Abstract
Copper(I)-based emitters show great potential for addressing the challenges of current organic light-emitting diode (OLED) technology. They can match current state-of-the-art phosphorescent materials for efficiency and can be tuned in color from red to blue. This paper gives an overview, describing examples of mono- and dinuclear Cu(I) complexes in terms of structures and properties. In particular, the modular structure of dinuclear compounds allows the independent tuning of emission color and solubility, making these materials perfect candidates for large area OLEDs produced from solution.
Export citation and abstract BibTeX RIS
1. Introduction
Organic electronics—particularly organic light-emitting diode (OLED) displays for smartphones and televisions—are becoming more prevalent in our everyday lives, due, in part, to their high-contrast capability and low energy consumption. Although OLED televisions currently have a high production price tag, as production quality improves both production and consumer costs will decrease. Area lighting, in contrast to bulb lighting, is yet another upcoming application specific to OLED technology, in which the illuminating lamp can be flexible and tailor-made. The main requirements for both of these applications is that they require efficient dyes for the transformation of current into light, and while most of the displays integrate efficient red and green dyes, there is still a need for a good and stable blue emitter.
The production process is an important step in the development of OLEDs. Currently, most OLED-producing companies employ vacuum deposition techniques, i.e. thermal evaporation at low pressure around 10–7 mbar, which is well established and produces OLED stacks with well-defined interfaces and high reproducibility. This process is used for many lighting applications, where 'tandem OLEDs' have three different color layers stacked on top of each other. Such a setup was found to raise the power efficiency of these devices while maintaining an unstructured active lighting area. However, vacuum processing is material intensive because the majority of the material is deposited on the vacuum chamber rather than the substrate. Preparation of OLEDs on large substrates, necessary for lighting panels and television screens, poses another challenge; production yields are low and therefore costly, because one defect alone can render the whole device unusable, and this is typically detectable only after the completed production process. Therefore, several new techniques of device preparation are being developed that are based on wet processing for the layering of the materials onto the device. This can be achieved, for example, via ink-jet printing or slot-die coating. Ink-jet processing can be used for digital printing of structured devices, while slot-dye coating is suitable for producing uniform layers in devices. Solution-processing tends to be easily scalable and therefore has the potential to be the most suitable way to fabricate large OLED devices.
The use of fast and material-efficient production techniques will allow novel devices that can be used as illuminating labels on products to attract and inform consumers visually, increasing consumption, in what is called 'smart packaging'. Smart packaging requires a mass producible and compatible device system to incorporate new functionalities like sensing and illumination. Sensors coupled to packaging can effectively inform about the quality of the product inside the packaging, for example, if the product has gone bad. Therefore, OLEDs are suitable for packaging that displays basic information about a product or readouts from incorporated sensors. To allow the growth of this sector as a third major application of OLEDs in everyday life, a solution-based process should ideally be combined with abundant materials that are suitable for real mass production outside the display market. One of cynora's main interests is in developing emitter materials that are suitable for emerging markets such as lighting and displays, as well as smart packaging.
2. Emitting materials
A very important part of device architecture is the emissive layer, which is a blend of a conducting material with the emitter. The emitter can be characterized by the emission color and by the efficiency with which it transforms excitons into light. Excitons are created during device operation when electrons and holes become bound. There can be two types of exciton, singlets and triplets. The singlet and triplet excitons are generally formed in a 1:3 ratio [1]. Those emitters that only use singlet excitons for emission (fluorescence) are relatively inefficient in devices [1], while those that can generate light from both types of excitons are more efficient. Two pathways exist for obtaining light from both kinds of excitons: (i) triplet harvesting (phosphorescence), where singlet excitons are transformed into triplets, which are then transformed into light; and (ii) singlet harvesting (TADF), where transformation of triplet excitons into singlet excitons is followed by light emission (figure 1, [2]).
Figure 1. Overview of three different emission processes: fluorescence (up to 25% internal efficiency), triplet and singlet harvesting (up to 100% internal efficiency). Scheme courtesy of cynora GmbH.
Download figure:
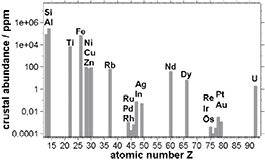
Standard image High-resolution imageEmitters with high efficiency and hence long-term stability can be achieved by introducing a metal ion into the dye, which enables a process of inter-system crossing (ISC), i.e. the transformation of a singlet into triplet excitons or vice versa. Many metals have been tested within OLED emitters, the most successful being iridium. Iridium(III)-based emitters can be tuned from red to blue and show high photoluminescence quantum yields (PLQY). Stacks built with iridium have advanced significantly since the turn of the century and show good performance when processed from vacuum. Despite its exceptional properties, iridium is a rare material and therefore a poor choice for future mass markets. Consequently, cynora has been actively researching iridium-free emitter materials, specifically materials based on copper. Copper materials have been shown to be suitable as luminescent emitters that participate in ISC, allowing highly efficient emission [3]. Copper has several advantages over iridium, in addition to being abundant (figure 2).
Figure 2. Abundance of selected elements in the Earth's crust: Cu (68 ppm) is several orders of magnitude more abundant than elements such as Ir (0.0003 ppm) and Pt (0.003 ppm), which are also discussed as potential luminophores in material sciences [4].
Download figure:
Standard image High-resolution imageThe electronic transitions that can occur in metal complexes are d–s transitions on metal centers, ligand-centered (LC) and inter-ligand (IL) transitions, and also charge transfer between the metal center and the ligands in a metal-to-ligand charge transfer (MLCT) or between two different ligands in a ligand-to-ligand charge transfer (LLCT) where luminescence is a result of the latter two types. Coinage metal complexes have received substantial attention since the 1990s because of their strong luminescence at ambient temperature in the solid state [5]. Unlike for Ir, Pt or Ru complexes, self-quenching does not occur for the metal complexes even for high concentrations or bulk samples. Cu(I) and Au(I) complexes exhibit high structural diversity and are emissive with several classes of ligands. Based on the involvement of MLCT, LLCT and metal-halide-to-ligand charge-transfer (M+X)LCT transitions in the emissive state, the emission color of these Cu(I) complexes can be easily tuned by systematic variation of the ligand system [6]. In a comparative study on mononuclear Cu(I), Ag(I) and Au(I) complexes with one di-imine ligand and an ancillary phosphine ligand, Hsu et al [7] found that the rate constants of S1-T1 intersystem crossing and T1-S0 radiative decay rates are significantly larger for Cu(I) complexes than for the Ag(I) and Au(I) analogues. This is due to a stronger spin–orbit coupling upon involvement of the metal d-orbitals in the lowest-lying electronic transition for the Cu(I) complexes. Hence, since the electronic configuration of copper has completely filled d-orbitals, transitions to the metal can be avoided, in contrast to iridium(III). This means that the potential of copper to reach stable and efficient emitters is very high, especially in the deep blue region, where the relevant energies usually lead to such internal limitations on iridium(III)-based emitter systems. Recently, Chihaya Adachi and co-workers demonstrated the feasibility of this approach by using carefully adjusted organic molecules, which had been processed using vacuum-deposition techniques, and these reached an external quantum efficiency of 20% [8]. While direct comparisons between copper complexes and purely organic molecules have not been employed yet, we believe that there are several advantages in favour of copper complexes. An intrinsic problem is the significantly lower inter-system crossing rate in the absence of heavier elements such as copper, meaning that particular care has to be taken in the adjustment of energy levels to achieve efficient singlet harvesting. This also leads to somewhat longer excited state lifetimes for the purely organic molecules, which affects efficiency roll-off and lowers device lifetime. Due to the mostly non-modular design of current organic singlet harvesting emitters, neither the relative differences between the S1 and T1 levels or the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels may be easily tuned. In addition to an easier adjustment of all relevant energy levels, the modular synthesis of copper(I) complexes allows for an easy tuning of emission colour from blue to red and good solubility in commonly used solvents. So far, organic molecules have mostly been employed in vacuum-processed OLED devices, due to their low solubility. Copper(I) complexes, on the other hand, may be modified easily for solution processing. Their synthesis is often performed by mixing readily available precursors under mild conditions.
This paper introduces two types of copper complex families that show good results as emitters in OLED devices. Mononuclear complexes and homo- and heteroleptic dinuclear copper complexes are presented. Their physical properties, i.e. solubility, emission wavelength and HOMO/LUMO energies, are shown to be tunable, based on modifying particular components of the complex. Moreover, we show that our copper-based materials exhibit singlet harvesting, also known as thermally activated delayed fluorescence (TADF), which leads to highly efficient OLEDs. To keep the overview presented in this paper concise, details regarding density functional theory (DFT) calculations, photophysics and coordination chemistry will not be given in full. Instead, references providing more detailed investigations are given. The lifetime measurements of these devices will be presented in a future publication, however, similar copper complexes have shown promising results, e.g. those of Thompson and co-workers reaching 700 h (100 cd m−2 [28]).
3. Mononuclear copper complexes
Mononuclear neutral copper(I) compounds are interesting luminescent materials with great potential for OLED applications [9]. Compared to cationic copper(I) complexes, which have been studied excessively during the last decades, neutral copper(I) compounds are preferential emitters due to their advantageous emission characteristics and the lack of mobile counterions, which can have a negative influence on the device performance [10].
A new class of mononuclear neutral copper(I) compounds has been investigated by cynora [11]. The complexes feature a general stoichiometry of [(N-N)Cu(P-P)] using PyrTet (5-(2-pyridyl)tetrazolate) as a chromophoric N^N ligand. A strong binding to the soft copper(I) center (HASB concept) is favored by this ligand, leading to a reduced oxygen sensitivity accompanied by high photoluminescence quantum yields [12]. Triphenylphosphine (PPh3), bis[2-(diphenylphosphino)phenyl]ether (DPEPhos), 9,9-dimethyl-4,6-bis(diphenylphosphino)xanthene (Xantphos) and bis[2-(diphenylphosphino)-p-tolyl]ether (PTEPhos) were used as neutral P-P ligands because they provide steric advantages and they are soft Lewis bases, favorable for coordination to the soft copper(I) ion. Four neutral copper(I) complexes were synthesized and examined with regard to their structure and their photophysical behavior (figure 3, left). The structure of complex 1 is shown exemplarily on the right-hand side of figure 3. As expected, the x-ray structure analysis reveals a tetrahedral coordination geometry, which is typical for mononuclear Cu compounds [13].
Figure 3. Scheme of synthesized neutral complexes (left) and x-ray molecular structure of complex 1 (right [14]).
Download figure:
Standard image High-resolution imageDFT calculations were performed on the neutral complex 1 to elucidate the nature of the frontier orbitals [11]. The HOMO is mainly restricted to the copper(I) center and the tetrazole moiety with small contributions from the pyridine N atom, while the LUMO is found primarily on the pyridine ring and to a lesser extent on the tetrazole ring, as can be seen in figure 4. In accordance with these results the absorption spectrum of complex 1 indicates metal-to-ligand charge transfer (MLCT) and in addition, intra-ligand charge transfer (ILCT) transitions. It should be noted that the phosphine ligands do not contribute to the charge transfer states in the case of the neutral complexes [11].
Figure 4. Calculated HOMO (left) and LUMO (right) of neutral complex 1 [14].
Download figure:
Standard image High-resolution imageThe room temperature powder emission spectra of complexes 1–4 are shown in figure 5 and a collection of the photophysical properties can be found in table 1. As can be seen from the spectra in figure 5, the emission band of these compounds is broad and unstructured, which is in accordance with the results from DFT calculations. The emission maxima of compounds 1–4 are in the green spectral region from 502–545 nm. The photoluminescence quantum yields and the emission decay times are in the range of 76–89%, as well as 17–27 µs in the solid state. The insignificant contribution of the phosphine ligands to the emissive states was also predicted by DFT calculations and is visible in the emission spectra, i.e. the similarity of their emission wavelengths. The influence of the phosphine ligands is related mostly to steric aspects by influencing the flattening distortion tendencies of the respective complexes, in which the ligands' effects are mostly reflected in the non-radiative rate constants knr (table 1, [11]).
Table 1. Photophysical properties of 1–4.
| Absorbtion |
Emission |
|||||
|---|---|---|---|---|---|---|
| complex | λmax [nm] (ε [104 M−1 cm−1]) | λmax [nm] | ϕem ± 0.05 | τave |
kr [s–1] | knr [s−1] |
| 1 | 260 (2.49), 273 (sh, 2.27), 339 (sh, 0.26) | 512 | 0.85 | 20.6 | 4.1 × 104 | 7.3 × 103 |
| 2 | 265 (2.16), 280 (sh, 2.15), 341 (sh, 0.56) | 510 | 0.78 | 19.9 | 3.9 × 104 | 1.1 × 104 |
| 3 | 278 (2.39), 341 (0.44) | 545 | 0.76 | 26.6 | 2.9 × 104 | 9.0 × 103 |
| 4 | 276 (1.92), 340 (sh, 0.50) | 502 | 0.89 | 17.8 | 5.0 × 104 | 6.2 × 103 |
aIn CH2Cl2, 10–5 mol L−1.
bIn solid state.
cPL lifetime is biexponential. For simplicity, a weighted-average lifetime was used ( ) and calculated by the equation
) and calculated by the equation  with Ai as the pre-exponential factor for the lifetime. See literature for details [11, 15].
with Ai as the pre-exponential factor for the lifetime. See literature for details [11, 15].
Figure 5. Powder emission spectra of complexes 1–4 at room temperature. Emission spectra were obtained after excitation at 350 nm [14]. Reproduced by permission of The Royal Society of Chemistry.
Download figure:
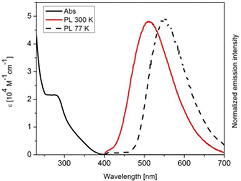
Standard image High-resolution imageTo clarify whether these emitting compounds are capable of singlet harvesting, complex 2 was investigated not only at room temperature, but also at 77 K. The emission spectra are shown in figure 6, showing a red-shifted emission maximum from 510 nm to 549 nm while cooling from 293 K to 77 K, as well as an increased lifetime from 19.9 µs to 111.5 µs, which is in accordance with the singlet harvesting model. Because of a thermal repopulation from the triplet state T1, the emission at 293 K originates from the higher-lying singlet state S1, while at 77 K the lower lying T1 is the emissive state [11]. In summary, neutral mononuclear complexes offer great potential for OLED applications because of their photophysical characteristics, i.e. extraordinary high emission quantum yields and short emission decay times, as well their ability to harvest all excitons via the singlet state due to singlet harvesting.
Figure 6. Absorption spectrum in dichloromethane at room temperature and emission spectra at 77 K and at 300 K of complex 2 [14]. Emission spectra were obtained after excitation at 350 nm. Reproduced by permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution image4. Dinuclear copper complexes
For copper(I) halide complexes many coordination motifs can be realized by varying the coordinating ligand as well as the molar ratio between ligand and metal salt [16]. The motifs [CuXL]4, [CuXL]∞, [(CuX)2L4] or [CuIL3] can be obtained when using imine-type ligands L, whereas many different kinds of structural motifs can be obtained by using mixtures of phosphine- and pyridine-type ligands, for example [Cu2X2L2(PR3)2] [16a, 17]. To achieve a straightforward synthetic pathway for the preparation of copper(I) halide cluster complexes, cynora's approach involves phosphane-substituted nitrogen-containing heterocylces, i.e. a combination of imine and phosphine functionalities [18]. In the case of 2-diphenylphosphino-pyridines (PyrPHOS-type ligands), these P-N ligands have been known for many years [19], however a large number of P-N ligands, having various nitrogen-containing N-heterocycles as a coordinating unit, have been synthesized (figure 7, [18]). The term PyrPHOS, which is limited to pyridine as heterocycle, may be extended to NHetPHOS. Metal complexes incorporating P-N ligands are favorable for tuning of their solubility and emission wavelength due to the properties of these ligands: soft electron donors and easily modifiable precursors [18, 20].
Download figure:
Standard image High-resolution imageThe synthesis of the dinuclear copper(I) complexes can be done by stirring the appropriate organic ligand together with the copper salt at room temperature. Pure materials can be obtained by repeated precipitation of the copper(I) complexes in apolar solvents followed by recrystallization. In many cases, high yields may be achieved.
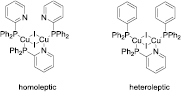
The complexes prepared by cynora were published recently within a study on dinuclear homoleptic copper(I) halide complexes with NHetPHOS-type ligands [18]. The basic structural motif [(CuX)2L3] includes a butterfly-shaped Cu2X2 core and three substituted NHetPHOS ligands (figure 9, left). One of the P-N-ligands acts as bridging ligand between the two copper(I) centers, while the two other ligands act as monodentate ligands, coordinating only via their phosphorous atoms. Each copper(I) atom features a pseudo-tetrahedral coordination environment. As shown below, this structural motif is also obtained for heteroleptic complexes [(CuX)2L2L'] where instead of the monodentate NHetPHOS ligands phosphines, phosphonites, phosphinites and phosphites may be introduced (figure 8 and vide infra, [21]).
Download figure:
Standard image High-resolution imageFigure 9. X-ray structure (left [22]) as well as HOMO (middle) and LUMO (right) frontier orbital plot of a typical dinuclear complex (shown here is complex 7-I exemplarily) based on DFT calculations. Reprinted with permission from [22]. Copyright 2013 American Chemical Society.
Download figure:
Standard image High-resolution imageThe nature of the emissive transitions responsible for the emission of this dinuclear copper(I) halide clusters has been investigated through DFT calculations, showing the HOMO mainly located on the copper halide core, while the LUMO is located on the bridging P-N ligand (figure 9). Consequently, these transitions are classified as metal-halide-to-ligand charge transfer transitions, (M+X)LCT [18, 21].
Knowing the localization of these frontier orbitals, it is possible to tune the emission color of these kinds of complexes by changing either the halides (lowering/raising the HOMO) or the ligands (lowering/raising the LUMO). In detail, moving from chloride to iodide results in a larger bandgap and is accompanied by a blue shift of the emission [18a, 21a]. Luminescence can be induced by altering the P-N-ligands, giving them different electronic properties, i.e. electron-rich or electron-poor heterocycles. Figure 10 presents the N-P-ligands investigated with respect to electron-rich (ligands 5–8) and electron-poor characteristics (ligands 9–13).
Figure 10. Electron-rich/-poor NHetPHOS-type ligands with varying electron richness/poorness used to change the emission wavelength of their corresponding homoleptic copper complexes [18].
Download figure:
Standard image High-resolution imageRoom-temperature powder emission spectra of the corresponding copper(I) iodide complexes 5-I–7-I and 9-I–13-I are shown in figure 11 and a collection of the photophysical properties can be found in table 2. In accordance with the assumption of a charge-transfer nature for the emitting state the emission spectra of all investigated complexes are relatively broad and unstructured, which correlates well with the results of the DFT calculations (vide supra). As discussed on the basis of these calculations, modifications of the ligands result mainly in altering of the LUMO energy of the corresponding complexes and therefore lead to a shift in the emission color. In detail, complexes composed of electron-rich N-containing heterocycles show an emission in the blue–green range (5-I–8-I), while those composed of electron-poor heterocycles are red-shifted (9-I–13-I) due to the stabilization and destabilization of the LUMO. Therefore, it is possible to obtain emission colors from blue (6-I, 458 nm) to deep red (13-I, 713 nm) by using different NHetPHOS ligands [18].
Table 2. Photoluminescence characteristics of complexes 5-I–13-I (neat powders, room temperature, λexc = 375 nm).
| compound | λmax [nm] | ΦPL |
|---|---|---|
| 5-I | 485 | 0.95 |
| 6-I | 458 | 0.10 |
| 7-I | 537 | 0.81 |
| 8-I | 522 | 0.96 |
| 9-I | 558 | 0.70 |
| 10-I | 572 | 0.33 |
| 11-I | 605 | 0.06 |
| 12-I | 657 | 0.16 |
| 13-I | 713 | 0.03 |
Figure 11. Neat powder emission spectra of complexes 5-I–7-I and 9-I–13-I at room temperature (λexc = 375 nm).
Download figure:
Standard image High-resolution imageThe photoluminescence quantum yields of these dinuclear compounds are able to reach values of up to 96% for the sky blue and green emitting complexes 5-I, 7-I and 8-I, while quantum yields of the more red-emitting complexes 9-I–13-I significantly drop, which is related to the energy gap law, i.e. a decrease in emission quantum efficiency with decreasing emission energy, since competing nonradiative processes become more relevant (table 2, [23]). The emission decay times are very short, in the range of 2–6 µs. This is already similar to those found for Pt(II) complexes, which have been used with good efficiencies in OLEDs, showing the great potential of our complexes for OLED applications [24].
The emission wavelength of NHetPHOS complexes can be tuned between blue (458 nm) and red (713 nm) by changing the heterocycles included in the N^P ligands. This kind of modification changes the energetics of the LUMO, which is located on the bridging NHetPHOS ligand and directly influences the (M+X)LCT transition energy. Subsequently, the monodentate P-ligands can be exchanged without significantly affecting the emission behavior. Therefore, besides engineering the band-gap energy and emission wavelength of these complexes, other functionalities may be introduced, e.g. adjusting the solubility of the complex (figure 12, [21]).
Figure 12. Schematic representation of heteroleptic PyrPHOS CuI complexes [21].
Download figure:
Standard image High-resolution imageThis has been proved for various examples by combining phosphines like aryl phosphines (14, 15, 18), aryl phosphites (19, 20), alkyl phosphites (21) or alkyl phosphines (17) with NHetPHOS ligands (figure 13). By using different monodentate P ligands the solubility in solvents such as toluene was enhanced, while, as expected, physical parameters such as band-gap, oxidation potential and emission decay time remained almost unchanged [21b].
Figure 13. Selected P ligands that have been introduced as monodentate ligands in heteroleptic dinuclear NHetPHOS complexes [21b].
Download figure:
Standard image High-resolution imageThe emission properties of these heteroleptic complexes remain mostly unaffected by using these different phosphines, which allows a tuning of the emission wavelength comparable to that for the homoleptic complexes. This has been proven by using triphenylphosphine as monodentate P ligand and different electron-rich (22–25, figure 14) and electron-poor P-N ligands (26, 27, figure 14), resulting in emission wavelengths from deep blue (451 nm) to yellow (558 nm), as can be seen in figure 15 [21a].
Figure 14. Electron-rich/-poor NHetPHOS-type ligands with varying electron richness/poorness used to change the emission wavelength of their corresponding heteroleptic copper complexes [21a].
Download figure:
Standard image High-resolution imageFigure 15. Neat powder emission spectra of complexes 22-I-PPh3–27-I-PPh3 at room temperature (λexc = 350 nm).
Download figure:
Standard image High-resolution imageAs explained above, this can be rationalized in terms of stabilization or destabilization of the LUMO energy of these complexes. Furthermore, color tuning was also accomplished by modifying the HOMO energy of these complexes by substituting the halides. Moving from chloride to iodide leads to a decrease of the HOMO energy, which is accompanied by an increasing band-gap; the emission color is thus shifted from yellowish orange to cyan blue. The color of the complexes with CN and SCN moieties may be sorted between Cl and Br. This order is correlated with a nephelauxetic parameter B, known from crystal field theory, where ligands are sorted according to their capability of forming covalent bonds with metals (I−1 > Br−1 > CN−1 > S-SCN−1 > Cl−1 > N-SCN−1). According to this, iodide acts as a strongly covalent ligand, which stabilizes the Cu2X2-unit. Consequently, the HOMO energy, located on this unit, is lowered [21b]. It should also be noted that these heteroleptic complexes show extraordinarily high photoemission quantum yields of up to 99% and short emission decay times below 5 µs (table 3, [21a]).
Table 3. Photoluminescence characteristics of complexes 2-I-PPh3–27-I-PPh3 (neat powders, room temperature, λexc = 350 nm).
| Compound | λmax [nm] | ΦPL |
|---|---|---|
| 22-I-PPh3 | 451 | 0.36 |
| 23-I-PPh3 | 468 | 0.72 |
| 24-I-PPh3 | 481 | 0.91 |
| 25-I-PPh3 | 498 | 0.99 |
| 26-I-PPh3 | 522 | 0.99 |
| 27-I-PPh3 | 558 | 0.86 |
In summary, cynora's homoleptic and dinuclear complexes offer a high modality towards adjusting not only the emission wavelength over the whole visible spectrum, but also the solubility in different organic solvents. Combined with their extraordinarily high emission quantum yields and their short emission decay times due to the singlet harvesting effect, these complexes offer enormous potential for use as emitting materials for next-generation printed OLEDs, which are outlined briefly in the next section.
5. Singlet harvesting in OLED devices built with Cu(I) emitting complexes
The materials described above have shown their suitability as emitters in OLEDs. To improve the device performance, cynora uses a combination of local and global design of experiments (DOE). This approach has allowed cynora to achieve high efficiencies in solution-processed OLED devices, achieving state-of-the-art data for phosphorescent systems.
One of the main elements in these systems is triplet–triplet annihilation (TTA), which does not occur for materials with efficient TADF [25]. Both processes are competing mechanisms for the depopulation of excited triplet states. A small singlet-triplet energy gap (ΔES1-T1) favors TADF over TTA. The reason behind this observation is thought to be the efficient reversed intersystem crossing between S and T states, which happens on a shorter timescale than the interaction of two molecules in triplet excited states. In addition, TTA is a bimolecular process, thus depending on the concentration of the triplet excitons, while TADF is a unimolecular process and per se independent of the concentration. A quantitative analysis of this phenomenon would require extensive experimental effort, as demonstrated by Baldo et al [26]. This eliminates the efficiency-limiting effect of TTA in the emitting layer (EML) and allows for higher doping concentrations, resulting in enhanced brightness, as often observed in OLEDs with Cu(I) emitters. As mentioned above, Yersin et al observed that the TADF mechanism allows harvesting of both singlet and triplet excitons in electroluminescent devices, leading to a theoretical internal efficiency of 100% [9, 27].
In representative OLEDs with green dinuclear emitters, high brightnesses of around 50,000 cd m−2 have been measured, accompanied by good device performance of around 50 cd A−1 [21], figure 16. In such devices, the layers for hole injection (HIL), hole transport(HTL) and emission (EML) have been deposited from solution on top of each other, while the electron transport layer (ETL), electron injection layer (EIL) and the cathode have been deposited from vacuum. By optimizing both the stack architecture and the production process, cynora has recently achieved record efficiencies of up to 73 cd A−1 with dinuclear copper complexes. These are the highest efficiencies reported for iridium-free, copper-based OLEDs, especially with a solution-processed EML and will be published separately. The lifetime measurements of these devices will also be presented in a future publication, however, similar copper complexes have shown promising results, with those from Thompson and co-workers reaching 700 h (100 cd m−2 [28]).
Figure 16. OLED-device with a NHetPHOS complex. Image courtesy of cynora GmbH.
Download figure:
Standard image High-resolution image6. Summary and outlook
In this paper, selected examples of cynora's emitters have been reviewed. It highlights mononuclear Cu(I) and dinuclear NHetPHOS Cu(I) complexes, which represent a good foundation for the design of next-generation OLED dyes. It has been demonstrated that they satisfy three main criteria for modern optoelectronic materials: (i) good performance, (ii) versatile processability and (iii) easily available starting materials.
Singlet harvesting emitter materials based on copper offer high quantum efficiencies, short emission decay times and tunable emission color. With their special emission principle they are able to harvest both singlet and triplet excitons in electroluminescent devices, making them perfectly suitable for OLED dyes. Since color and solubility may be tuned independently from each other, the materials can be adapted to a variety of requirements of wet processing. The use of appropriate host materials in combination with a matching stack architecture has resulted in high device efficiencies, at the level of currently used phosphorescent emitter systems.
These classes of materials represent a powerful set of building blocks for future OLEDs, both for vacuum- and solution-based processing. Additionally, new functionalities can be introduced, enabling autocatalyzed crosslinking reactions, making this system ideal for next-generation OLED technology.
Acknowledgements
We thank Professor Stefan Bräse (KIT) for supporting this research. We acknowledge the Deutsche Forschungsgemeinschaft (project B2 of SFB/TRR 88) and the German Federal Ministry of Education and Research for support through the funding programs cyCESH and cyFLEX.