Abstract
Due to the unique properties of silver nanoparticles there is growing interest in their applications. Current trends in nanotechnology are focused on developing a new technique to synthesize nanoparticles using biological methods associated with the use of plant extracts, fungi, bacteria or essential oils. These methods are a promising alternative to conventional approaches which can minimize the use of hazardous substances. The silver nanoparticles synthesis using red tea infusion as a reducing and stabilizing agent and their characteristics have been described. Total antioxidant capacity using DPPH radical and total content of phenolic compounds by Folin–Ciocalteau method were measured in tea infusion. Synthesis of silver nanoparticles was carried out using chemical reduction at various temperatures. Furthermore, the effect of tea infusion volume added to reaction mixture on nanoparticles' properties was investigated. Finally, nanosilver suspensions were characterized by UV–vis spectrophotometer, dynamic light scattering (DLS) scanning electron microscope (SEM) and transmission electron microscope (TEM). Moreover, phytotoxicity of silver nanoparticles was determined using Phytotestkit microbiotest.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology is one of the most modern and thriving fields of science which allows for manipulation at the atomic and molecular scale. Within the size range of 1–100 nm, nanostructures are characterized by new properties, i.e. physical, chemical and biological, of individual atoms in comparison to their bulk materials [1–3]. Due to a very small size, nanoparticles possess an immense fraction of surface atoms per unit volume causing high chemical activity. Because of the unique properties of nanoproducts, (chemical stability, catalytic, antibacterial, anti-viral and antifungal properties) there is growing interest in their applications, especially in optic, electronic and chemical industries as well as in cosmetology [4–6].
Generally, a number of approaches are available for metal nanoparticles preparation using chemical and physical methods. Unfortunately, these processes are quite expensive and may involve use of hazardous substances which are potentially dangerous for the environment. In the chemical reduction process, silver salts such as AgNO3 or less frequently AgBF4, AgPF6, AgClO4 are reduced in an aqueous solution to form silver nanoparticles. Sodium borohydride (NaBH4) is the most commonly used as a reducing agent in this method. Additionally, stabilizing agents such as polyvinylpirrolidone (PVP), polyvinyl alcohol (PVA) or sodium dodecyl sulfate (SDS) can be added to prevent agglomeration of nanoparticles [7–9].
Chemical reduction process of metal ions entails three steps. Firstly, during reduction of metal ions, free metal atoms are created, which subsequently collide with each other to form a stable 1–2 nm nuclei. This stage determines the monodispersity of obtained nanoparticles. Concentration of the reducing agent, temperatures and reduction potential of the reaction are important because they affect the initial concentration of nuclei. In the second stage, further reduction of metal ions occurs until all the metal ions are consumed. The last stage is stabilization of obtained nanoparticles through addition of a suitable agent. The morphology and stability of nanoparticles can be controlled through adjusting a process parameters such as concentration of metal salt, content of reducing and stabilizing agents and temperature [4, 10–12].
Nowadays, the focus of nanotechnology research has significantly shifted from traditional applications and evolved into green processes. This method can employ natural plant extracts, essential oils, fungi or bacteria for production of nanoparticles [13–15]. Green synthesis is focused on the minimizing the use and generation of hazardous chemicals such as formaldehyde or sodium borohydride—commonly used as a reducing agent in conventional methods. Moreover, in eco-friendly processes, plant extracts are also used as effective stabilizing agents of nanoparticles suspension which eliminate the use of other chemical compounds during production of nanoparticles [9, 16, 17].
Considering the vast reduction potential of naturally occurring substances in plant extracts, there are many reports proving their use in biological green technique. A large number of plant materials are found in scientific literature on biosynthesis of nanoparticles, particularly Aloe leaves, Cymbopogon citratus, Cordia dichotoma, Chamaemelum nobile, Xanthium strumarium L. or lamb's quarters [14, 18–21]. There is still a need to find an economically acceptable and environmentally friendly, natural reducing source for green nanoparticles synthesis which has not been studied yet [22–24].
In recent times, great attention has been devoted to different types of tea leaves which also possess compounds responsible for metal ions reduction. Tea leaves contain more than 2000 components including polyphenols, alkaloids, vitamins, polysaccharides, lipids and pigments. Closet attention has been paid to the polyphenols which include catechins, theaflavins, tannins and flavonoids. These compounds affect the flavor of tea and show potential health benefits arising from their antioxidant activity. Because of the chemical structure of polyphenols which allows for electron delocalization, a high reactivity to quench free radicals is exhibited. Total polyphenols content (TPC) constitutes about 35% of the weight of dried leaves [25–27]. The most important compounds in polyphenols family are the catechins which make up to 30% of the tea leaves while the flavones and flavonols in total do not exceed 4%. Tea is also recognized as an essential source of xanthine (caffeine, theophylline, theobromine), pectin, protein compounds (glutelin, albumin, enzymes and the amino acid theanine), mineral salt components (manganese, fluoride, aluminum, calcium, zinc, sodium, potassium), vitamins (A, B1, B2, C, E and K), pigments (chlorophyll, xanthophyll, carotene, thearubigins and theaflavins) [26, 28, 29].
Depending on the processing method of leaves, tea can be classified into three main types: green, black and red tea, characterized by different quality properties including aroma, taste and color, and appearance. Red Pu-erh tea used in the study is a variant of aged dark tea, which passed through the fermentation and oxidation processes. All types of Pu-erh tea derive from green tea Maocha of the species Camellii Sinensis that grows in the mountains in Yunnan province, China. Typically, the production of Pu-erh tea is composed of several steps. At first, wet leaves are spread out in the Sun after which they wither. After the drying process, the oxidation phase begins. Then, dry leaves are broken down in layers in the fermentation room and they are maintained in a suitable temperature and high humidity. Then the additional process of fermentation follows; it lasts several days. Interestingly, in China, the Pu-erh tea, due to the color of infusion, is called 'black tea' [29, 30].
In this study we attempted to obtain silver nanoparticles synthesis using red tea infusion as a reducing and stabilizing agent under varying process conditions such as temperature, reaction time and quantity of added reducing-stabilizing agent. The synthesis was continuously monitored by UV–vis spectrophotometer, which allows to confirm the presence of silver nanoparticles. The next research step was to measure total antioxidant capacity using 1,1-diphenyl-1-picrylhydrazyl (DPPH) radical and total content of phenolic compounds using the Folin–Ciocalteau method. Ultimately, properties of achieved nanoparticles suspensions such as the average particle diameter and their morphology were determined using dynamic light scattering (DLS), scanning electron microscope (SEM) and transmission electron microscope (TEM), respectively.
2. Experimental
2 g of dry tea leaves were extracted in 100 ml of water at 80 °C. The mixture was covered with a watch glass and allowed to infuse for 30 min. Subsequently, the obtained infusion was filtered through filter paper and then stored at 5 °C. The filtrate was used after 1 week.
The synthesis of silver nanoparticles was achieved using classical chemical reduction of silver ions. For this purpose, different volumes of tea infusion were added to 50 ml of 250 ppm AgNO3 (POCH Poland S.A.) aqueous solution ranging between 2–15 ml, depending on experiment. The mixtures were stirred using magnetic stirrers. As a result, brown solutions were created, proving the formation of silver nanoparticles. Furthermore, their formation was also periodically monitored using recording UV–Vis spectra with Evolution 201 spectrophotometer (Thermo Scientific) over the wavelength range from 300 to 750 nm in cuvettes with an optical path length of 10 mm. Measurements were performed at room temperature, using double-distilled water as a blank. Several syntheses of AgNPs at different reaction temperatures from 10–50 °C in 10 °C intervals were conducted. Moreover, the stability of the obtained silver suspension was examined. The average particle size and size distribution of silver nanoparticles were measured with dynamic light scattering method (DLS) using a zetasizer nano ZS apparatus (Malvern Instruments Ltd.). All samples were measured three times and the measurements were performed at room temperature. The morphological and topographical analysis of obtained silver nanoparticles were carried out with transmission electron microscopy (TEM) and scanning electron microscopy (SEM) using JEOL JSM-7500 F equipped with a snap EDS-INCA PentaFETx3. All samples were first coated by 20 nm layer of chromium.
In this study antioxidant activity of red tea leaves infusion was assessed using DPPH assay according to slightly modified of Brand-Williams et al method [31]. This method is based on the reduction of DPPH reagent which is a stable free radical. The free radical with an odd electron gives a maximum absorption band at 517 nm and has a deep purple color in the ethanol solution. In the presence of an antioxidant substance, DPPH radical is reduced, which causes its color to change, becoming colorless or light yellow. These changes can be monitored spectrophotometrically. The presence of reduced form of the indicator results in absorbance decrease, which is proportional to the remained amount of DPPH in the solution [31].
1,1-diphenyl-2-picryl-hydrazyl (DPPH) was purchased from Sigma-Aldrich. 0.5 mM alcoholic solution of DPPH was prepared through dissolving 19.71 mg of DPPH in 100 ml of ethanol. The solution was diluted to the absorbance of approx. 0.9. Firstly, the absorbance of free radical solution was measured by adding to cuvette 2 ml of DDPH and 200 µl of ethanol (control). Subsequently, tested solution consisting of 2 ml of DPPH solution and 200 µl of diluted tea infusion was prepared and allowed to stand for 10 min at room temperature. After that, the absorbance was measured at 517 nm. For each specimen, the measurement was performed 3 times and the divergent results were rejected. The results were expressed as percent inhibition and were calculated according to the equation:
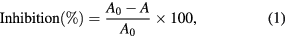
where A0 is the absorbance of control sample and Ā is the average value of absorbance of test sample [31].
The Folin–Ciocolteau (F–C) assay is a colorimetric method used to determine total phenols content (TPC) in samples. This method relies on electron transfer in an alkaline medium between phenolic compounds and Folin–Ciocolteau reagent, resulting in blue color formation proportional to the concentration of phenols. Total content of analyzed compound is quantified by measuring the absorbance of solution using a spectrometer at 765 nm. Gallic acid (acros organics) was used as a standard for spectrophotometric measurements. Total phenol values were expressed as gallic acid equivalents (GAE, mg g−1) based on the calibration curve. Briefly, 5 mg ml−1 of gallic acid solution was prepared as a working solution. The calibration standard solutions at different concentrations were appropriately diluted to 0.05; 0.15; 0.25; 0.35; 0.5 mg ml−1. Firstly, the absorbance of calibration solutions was measured by adding to cuvette 20 µl of calibration standards, 1.58 ml of double-distilled water and 100 µl of F–C reagent. The samples were allowed to stand for 3 min, and after that 300 µl of saturated sodium carbonate solution was added. Next, standards were mixed and placed for 30 min in the thermostat set at 40 °C. The measurements were carried out at 765 nm against blank sample without gallic acid. Subsequently, tested solution samples were prepared in manner identical to the calibration curve samples. The results were expressed as gram gallic acid equivalent/gram of dry tea leaves (g GAE g−1). Additionally, measurements were conducted in triplicates [32].
The phytotoxicity of silver nanoparticles was determined based on Phytotestkit microbiotest which allow for direct length measurements of roots and shoots in special transparent test containers, with the aid of image analysis. In the present experiment, 10 ml of AgNPs suspension at 12.5, 25.0 and 50.0 ppm (in comparison to reference solution—deionized water) evenly distributed on a thick filter paper surface placed on top of a parafilm sheet and a foam pad. The seeds of Sinapis alba and Sorghum saccharat were located at equal distance on a black filter paper placed on the top of the spiked white filter paper. After closing the test plates with their transparent cover, they were placed vertically in a holder for incubation at 25 °C for 3 d. Pictures of the test plates were taken at the end of the exposure period and measurements were performed with the aid of an 'Image Analysis program', such as the Image J program. Inhibition of root and shoot growth was calculated according to formula:

where A is average number of germinated seeds and average root and shoot length in the control and B is average number of germinated seeds and average root and shoot length in the concentration of the tested silver nanoparticles.
Taking into account the potential use of metallic nanosuspension for agrochemical applications, an experiment was conducted to investigate the stability of nanoparticles according to pH of the medium. Nutrient availability is greatly affected by a soil pH, whose optimal range for most plants is between 4 to 8. The obtained nanoparticles suspension was suspended subsequently in phosphate/citrate buffers prepared in accordance with Polish standard by mixing appropriate volume of 0.2 mol l−1 disodium hydrogen phosphate and 0.1 mol l−1 citric acids [33]. Studies consisted of suspending 1 ml of the analyzed metallic nanosuspension in 2 ml of a buffer solution. Next, the UV–vis spectrum was measured after 24 h of incubation time.
3. Results and discussion
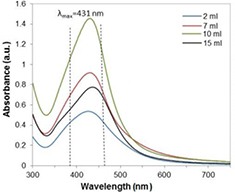
During reaction the color of the mixture turned from colorless to dark brown which is associated with silver nanoparticles formation as a result of the surface plasmon resonance (SPR) phenomenon. The size of silver nanoparticles can be characterized by the shape and peak position of the absorption spectrum. Silver nanoparticles formed through adding 10 ml of tea infusion to the reaction mixture showed the highest absorbance intensity, whereas the sample containing 2 ml display a low intensity broad absorption band (figure 1). The position of maximum absorption for suspensions containing 10 ml of tea is shifted to a shorter wavelength; consequently, the size of the particles are expected to be smaller. It is noted that addition of 15 ml of reducing-stabilizing agent causes a marked decrease in absorbance intensity. A single-peak absorption spectrum corresponds to the spherical shape of silver nanoparticles. Moreover, broad absorption peak determines dispersity of the nanoparticles, where it is attributed to polydispersity.
Figure 1. The UV–Vis spectra of the reaction mixture with different volume of tea infusion.
Download figure:
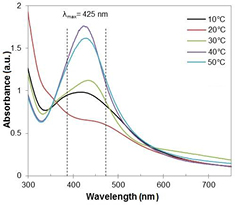
Standard image High-resolution imageTemperature is the main factor affecting the chemical reaction rate. The role of reaction temperature in the formation of silver nanoparticles was presented in figure 2. The effect of the reaction temperature was systematically investigated through adjusting between 10 and 50 °C. The solution temperature was reduced using an ice-bath or increased in the heating mantle. To minimize the impact of other experimental parameters, such as concentration of silver nitrate and the volume of added tea infusion, the above values remain constant. The temperature effect on the band intensity is pronounced. High temperatures have a significant effect on activity of tea infusion in reducing of silver ions, which was confirmed by the increase in the intensity of the absorption band. Furthermore, it is noted that with temperature increasing, the strongest absorption bands gradually shift to a shorter wavelength. This means that the nanoparticles size decreases with temperature.
Figure 2. The UV–Vis spectra of the reaction mixture recorded as a function of temperature.
Download figure:
Standard image High-resolution imageThe impact of time on the stability of nanoparticles was tested after four weeks (figure 3). It was observed that the intensity of the absorption increases in accordance with reaction time. Furthermore, there is a significant difference in the absorption spectra at the different reaction stages. The maximum absorption occurs initially at 440 nm while after 2 weeks, the highest intensity was obtained at 430 nm. It may indicate that the formed silver nanoparticles have a smaller diameter. In addition, the UV–vis spectra show broad absorption peak suggesting that the obtained silver suspension has a non-uniform size distribution of nanoparticles. AgNPs were stable for three weeks after completion of the reaction, and after that time the significant decrease in absorption intensity was observed.
Figure 3. The UV–Vis spectra of the reaction mixture recorded as a function of time.
Download figure:
Standard image High-resolution imageThe number distribution of silver nanoparticles is depicted in figure 4. It was observed that the size distribution of AgNPs ranges from 25 to 85 nm and the calculated average particle size is 53 nm. Furthermore, the amount of particles with diameter around 45 nm is rather larger than the amount of bigger particles, which is in line with the results of the absorption spectra. Moreover, the presence of narrow peak refers to monodispersity of the obtained suspension.
Figure 4. The measurements of particle size distribution of silver nanoparticles obtained at 40 °C.
Download figure:
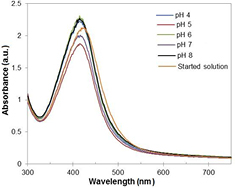
Standard image High-resolution imageThe stability of obtained silver nanosuspension at various pH conditions was evaluated (figure 5). It is noted that suspended silver nanoparticles were well-dispersed in the presence of prepared buffer solutions and remain stable at entire pH range. The UV–vis absorbance measurements did not show any significant differences in maximum absorbance values for all samples and slight difference in absorbance peaks position suggested that the average diameter of AgNPs remained unchanged. Moreover, no visible changes in the characteristic color of nanoparticles or the formation of aggregation were observed in each samples.
Figure 5. The UV–vis spectra of AgNPs after stability test (reaction temperature: 40 °C).
Download figure:
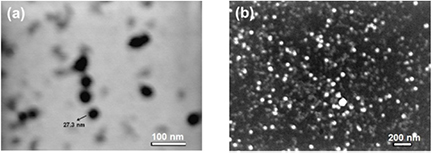
Standard image High-resolution imageFigure 6 shows the TEM and SEM image of AgNPs synthesized at 40 °C which predominated by almost spherical shapes ranging from 12 to 35 nm. Most of the silver nanoparticles have smooth edges. However, larger silver nanoparticles were observed as well, which is probably due to aggregation or clustering of the nanoparticles.
Figure 6. Transmission (a) and scanning (b) electron micrograph of AgNPs synthesized at 40 °C.
Download figure:
Standard image High-resolution imageAdditionally, scanning electron microscopy analysis demonstrates a high concentration of obtained silver nanoparticles. It was clearly visible that relatively spherical, regular and uniform particles were formed. Besides, the figure depicts that the resultant particles are very fine with average sizes from 15 nm to 100 nm. The nanoparticles were not in direct contact, based on the stabilizing effect of tea infusion. The presence of larger silver particles may be associated with the aggregation of the smaller ones. Furthermore, homogeneously distributed nanosilver particles can be seen on the entire surface.
Table 1 gives the percent inhibition (%) of red tea infusion with various dilutions. Antioxidant effects arise from the ability to donate hydrogen ions to stabilize the free radicals. A high inhibition percent indicates a strong antioxidant activity which is inversely proportional to the remaining DPPH quantity in the tested solution. It has been observed that dilution of tea infusion does significantly affect the scavenging activity of DPPH radicals. Considering the dilution, tea infusion is characterized by relatively high antioxidant capacity such as 48.7 ± 0.11% and 30.3 ± 0.28% for dilutions 1:5 and 1:10, respectively.
Table 1. Antioxidant activity of red tea infusion.
| Dilution | Absorbance | Inhibition (%) | |
|---|---|---|---|
| A0 | Ā | ||
| 1:5 | 0.756 | 0.388 | 48.7 ± 0.11 |
| 1:10 | 0.756 | 0.527 | 30.3 ± 0.28 |
The calibration curve showed linearity for gallic acid with correlation coefficient, R2 equal 0.996. The total polyphenol content in water infusion of Pu-erh tea, using the calibration curve, was 87.5 ± 0.28 mg of gallic acid equivalents/g. Due to the presence of hydroxyl group, the phenols exhibit strong scavenging ability for DPPH radicals. It is known that there is a strong correlation between antioxidant activity and total polyphenol content.
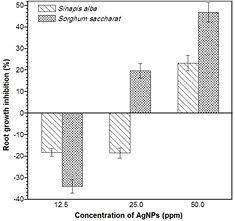
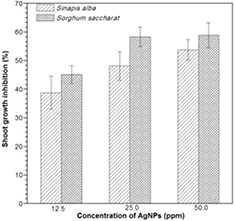
Figures 7 and 8 show the seedling growth of the test species exposed to AgNPs expressed as a percentage of the growth inhibition of root and shoot, respectively. It was observed that the growths of Sinapis alba and Sorghum saccharat. were adversely effected by increasing the silver nanoparticles concentrations. The symptoms of phytotoxicity were shown both in roots and in shoots; however, shoot growth showed more inhibition effects than root growth in all concentrations. Sinapis alba and Sorghum saccharat roots exposed to silver nanoparticles had a stimulatory effect at the lowest dose showed. Root growth inhibition were −18.19% and −33.94%, respectively. The intermediate AgNPs concentration (25 ppm) did not show a significant effect on the growth of Sinapis alba root, as opposed to Sorghum saccharat. seeds, where an increase in growth inhibition was observed. Shoots growth responded negatively to silver nanoparticles exposure. All three doses elicited this response, however the inhibitory effect was strongest for Sorghum saccharat seeds (58.89% for 50 ppm dose). Generally, plant shoots was significantly more sensitive to exposure to silver nanoparticles than roots of model plant.
Figure 7. Effect of silver nanoparticles on the roots growth rate of Sinapis alba and Sorghum saccharat.
Download figure:
Standard image High-resolution imageFigure 8. Effect of silver nanoparticles on the shoots growth rate of Sinapis alba and Sorghum saccharat.
Download figure:
Standard image High-resolution image4. Conclusion
The present work reveals a simple, cost-effective and ecofriendly method for silver nanoparticles preparation using red tea infusion as a reducing and stabilizing agent. AgNPs were successfully synthesized using chemical reduction method. The reduction of silver ions to nanoparticles were confirmed through color change and monitored quantitatively by UV–vis spectroscopy. DLS and UV–vis spectroscopy as well as SEM and TEM were used to characterize the dispersity and morphology of nanoparticles. Further characterization with DLS, SEM and TEM analysis showed the spherical, monodisperse AgNPs with average particle sizes ranging between 12–35 nm. In addition, the properties of silver nanoparticles are incorporated within various amounts of tea infusion added during reduction process of Ag+ . Silver nanoparticles synthesis proceeded most efficiently using the 7 and 10 ml of red tea infusion. Moreover, chemical reduction of silver ions to nanoparticles proceeded best at higher temperatures (40 °C and 50 °C). However, further investigations were needed to identify the scaling-up usage of tea infusion in silver nanoparticle production and its applications. Moreover, it was shown that the total polyphenol content in the Pu-erh tea was correlated with its antioxidant properties. The results have demonstrated the phytotoxicity of silver nanoparticles to Sorghum saccharat. and Sinapis alba after a 3 d of exposure. Results showed that inhibition of plant growth was evident after exposure to high concentrations (50 ppm).
Acknowledgments
The authors would like to thank The National Centre for Research and Development (Grant no: LIDER/037/481/L-5/13/NCBR/2014) for providing financial support to this project.