Abstract
Gold nanoparticles (AuNPs) in polymeric polyacrylamide (PAAm) matrix were synthesized using conventional heating and autoclaving thermal techniques. The synthesized Au/PAAm nanocomposite was characterized using UV–vis spectroscopy and high-resolution transmission electron microscopy. The size of the synthesized particles was approximately 6.37 nm and 8.19 nm with the conventional heating and autoclaving thermal techniques, respectively. Electron diffraction x-ray spectroscopy and the Fourier transformation infrared spectroscopy were used for the composition and elemental studies, which confirmed that the Au metallic atoms were synthesized and embedded within a PAAm matrix via a coordination bond between the carbonyl (–CONH2) group and the metallic NPs. X-ray diffraction confirmed the crystalline nature of the fabricated AuNPs with face centered cubic of nanocrystals. The catalytic activity of the as-prepared Au/PAAm nanocomposite for the reduction of 4-nitrophenol to 4-aminophenol was studied in the presence of sodium borohydrate. The synthesized AuNPs had an effective catalytic activity; the smaller NPs synthesized NPs with the conventional heating technique had a higher reaction kinetic rate in comparation with those synthesized with the autoclaving technique. Therefore, the Au/PAAm nanocomposite can be widely used as an eco-friendly, non-toxic, a fast and cost-effective product to remove versatile organic pollutants such as aromatic nitro compounds.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology, a new fascinating emerging technology, has several potential applications in different fields such as medicine, pharmacy, agriculture, and industries. The nanoparticles (NPs) have a small size of <100 nm, and thus possess numerous properties that are different from bulk/macroscopic systems [1, 2]. For example, quantum dots are clusters of atoms less than tens of nanometers in size. In nature, these quantum dots emit different colors depending on their specific particle size. As the particle size decreases, the ratio of surface area (SA) to volume (V) increases rapidly. Therefore, NPs with large SA possess different, unique properties that can have widespread applications in branches such as electronics, composite material, chemical waste treatment, and catalysis industry. During vital chemical transformations, catalysis plays an essential and central role [3, 4]. Several methods have been used for the synthesis of metallic NPs such as chemical, sonochemical [5], laser ablation [6], γ-rays irradiation [7], microwave [8, 9], and hydrothermal [10] methods. Polyacrylamide (PAAm) is a water-soluble, non-toxic, long-chain synthetic polymer. It can be used in electrophoresis, as a food additive, for oil recovery, for preventing soil erosion, for water waste treatment, in cosmetics, in paper making, and for textile manufacturing [11].
The conventional thermal heating reduction technique is a primitive, widespread technique that is used for the reduction of metallic salts to metallic NPs (e.g. Ag, Au, Cu, Pt, Ti, and Pd NPs). This could be achieved using chemical reducing agents, such as ethylene glycol [12, 13], hydrazine hydrate [14, 15], and glucose [16], in the presence of PAAm as a capping (a stabilizing agent). The hydrothermal technique, another oldest technique, was defined by Roy [17] as the synthesis of NPs from a component of solid phases, involving water as a catalyst and at occasional instances where the synthesis reaction was conducted at elevated temperature (>100 °C) and pressure (>1 atm) [17]. The main advantages of hydrothermal technique are as follows: (1) it facilitates the reaction between inorganic solids which cannot react at an ambient room temperature; the elevated temperatures (>100 °C) break the chemical bonds of the reactants, enabling the new atoms to migrate through the reaction medium before the chemical reaction yields the final products; (2) the high pressure (>1 atm) created using an autoclave facilitates fast growth rates that are proportional to the rapid diffusion rates [18]; (3) the stretching of the polymeric matrix makes it more accessible for embedding and stabilizing the fabricated NPs [19].
Catalytic reagents can reduce not only transformation temperatures and reagent-based wastes, but also enhance the specific selectivity of a reaction to avoid the unwanted side reactions that may lead to green technology. The most common types of catalysis are homogenous, heterogenous, and enzymatic catalysis. Enzymatic catalysis is efficient in nature, whereas both homogenous and heterogeneous have their own merits and demerits according to the reaction system [20]. It is advantageous to use nano-catalysts as they can undergo both homogeneous and heterogeneous catalysis, allowing rapid recovery and excellent product yield of chemical transformations. Metallic nanocatalysts act as heterogeneous catalysts, having a very high catalytic activity and selectivity for specific reactions. Nano-catalysts usually take part in reduction reactions [21, 22], oxidation reactions [23], electrochemical reactions [24–28], and coupling reactions [29].
This study aimed at synthesizing AuNPs via a simple and inexpensive method using two different techniques, conventional thermal heating and hydrothermal autoclaving techniques. To investigate whether the as-produced AuNPs have widespread industrial applications or not, the reduction in their catalytic effect on 4-nitrophenol in the presence of excess sodium borohydride (NaBH4) was studied to confirm its efficacy in the removal of 4-nitrophenol from the environment as a waste product.
2. Materials and methods
2.1. Materials
Tetrachloroauric acid tetrahydrate salt (HAuCl4 · 4H2O, 99.5%), 4-nitrophenol, and NaBH4 were obtained from Sigma-Aldrich, St. Louise, CA, USA. PAAm was supplied from Merck, Germany. All the chemical reagents used were of analytical grade and did not require further purification. Highly purified de-ionized (DI) water was used as a solvent for all the prepared solutions.
2.2. Synthesis of AuNPs in PAAm
In a typical procedure, PAAm (1.0 g) was dissolved in 100 ml DI water using a magnetic stirrer at room temperature (25 °C) for 3 h. HAuCl4 · 4H2O salt was serially diluted (0.1, 0.5, 1.0, 2.5, 5, and 10 mM). In the conventional thermal heating technique, 22.5 ml (fixed volume; 1%, wt%) of PAAm was mixed with 2.5 ml of different HAuCl4 · 4H2O molar concentrations with continuous stirring for 1 h at 70 °C, as previously reported by Jie Bai et al [30]. The HAuCl4 · 4H2O solution was added dropwise to the PAAm solution until complete dissolution. The formation of AuNPs was confirmed by the observed color change of the different diluted solutions; colorless: 0.1 mM, faint pink: 0.5 mM, pink: 1.0 mM, faint red: 2.5 mM, red: 5 mM, and blood red: 10 mM. In the autoclaving heating (AH) technique, the same mixtures with different concentrations were transferred to an autoclave boiling tube and kept in an autoclave at 15 psi (103 kPa) pressure and 121 °C temperature for 1 h of irradiation time. Subsequently, the prepared solutions were kept at room temperature to cool down and centrifuged at 6000 rpm. After washing three times with DI water to remove unwanted impurities, the synthesized nanoparticle precipitate was stored for further investigations.
2.3. Characterization
The morphology of the as-prepared AuNPs capped by PAAm was studied using a high-resolution transmission electron microscope (HRTEM) and selected area diffraction angle (SAED) JEOL JEM-2100 microscope, operating at 200 kV. UV–visible spectroscopic measurements were performed using Thermoscientific 200 UV–vis, scanning a wavelength 200–900 nm. Energy dispersive x-ray (EDX) was used for compositional and elemental studies carried out using a JOEL scanning electron microscope equipped with EDX detection and operating at 40 kV. In addition, the Fourier transform infrared (FTIR) spectra were recorded by FTIR ThermoFisher Nicolets IS10 using KBr pellet technique, where the transmittance spectra were recorded between 400 and 4000 cm−1. The crystalline structure of the samples was examined using an x-ray diffractometer (PANalytical X'Pert Pro Multipurpose diffractometer) by exposing powder samples to Cu-Kα x-ray radiation (λ = 1.5418 Å) at 45 kV and a current of 30 mA.
2.4. Catalytic activity of AuNP catalysts in 4-nitrophenol reduction reaction
To study the catalytic efficacy of AuNPs, the reduction of 4-nitrophenol was chosen as a model system to quantify the catalytic activity. In a typical procedure, 2 ml 4-nitrophenol (0.08 mM) was added to a 3 ml quartz cuvette (1 cm path length) and mixed with 0.5 ml freshly prepared NaBH4 (0.08 M). Subsequently, 0.3 ml of as-prepared AuNPs was added; all the reactions are known to follow pseudo-first-order decay kinetics. On addition of AuNP catalysts, the reaction progress was monitored at every 0.5 min with a UV–vis spectrophotometer in the range of 200 and 700 nm at 25 °C. The original absorption peak of 4-nitrophenol is centered at approximately 317 nm, which shifts to 400 nm after the addition of the NaBH4 solution due to the formation of p-nitrophenolate ions under alkaline conditions.
3. Results and discussion
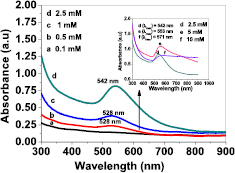
In order to compare the efficacy of the fabricated NPs either by the conventional thermal heating technique or hydrothermal autoclaving technique, the rapid and easy synthesized AuNPs were characterized by UV–vis absorption spectra, which is a successful tool for detecting the formation of NPs stabilized by PAAm polymeric matrix through the formation of a surface plasmon resonance (SPR) band [31–33]. The synthesized Au/PAAm nanocomposite displayed the specific characteristic SPR band in the spectral range of 520–600 nm; the synthesized particle size and the intensity of SPR band is directly proportional to the density of the synthesized NPs [34]. The characteristic SPR position is dependent on the Au(III) NP shape, size, structure, dielectric properties, and the embedding medium for the NPs that were formed according to the thermal decomposition of [HAuCl4]3+ salt.
According to Mie's theory, the red shift of SPR band is attributed to the formation of large NPs [35–37]. The UV–vis absorption spectrum (figure 1) of Au/PAAm prepared by conventional thermal heating method shows a gradual increase in the absorption spectral intensity with a red shift peak (from 525 to 541 nm) with the increasing [HAuCl4]3+ salt molar solution concentration from 0.1 to 10 mM. This indicates that large NPs were formed, implying that more nuclei can be synthesized in this reaction system by increasing the [HAuCl4]3+ salt concentration (0.1–2.5 mM). In addition, the formation of large NPs could be attributed to the increase in the effective collision number due to the supplied thermal heating energy in the reaction system [38]. Figure 1 shows that the polymeric PAAm matrix (capping agent) was not able to stabilize the reduced Au(0) NPs with the increasing Au salt concentration (5–10 mM) and it was accompanied with a more broader bandwidth, indicating the formation of NP clusters and agglomeration [24, 39, 40]. With the increasing Au(III) concentration, the formed nuclei in a colloidal solution are characterized by a large size and smaller separation distance. This increases the probability of collision between them enhancing the growth of the particles and finally leading to agglomeration and clustering [35].
Figure 1. UV–vis spectra of gold nanoparticles synthesized by the conventional thermal heating method, [HAuCl4]3+ salt concentration was 0.1–10 mM and polyacrylamide was 1 wt%.
Download figure:
Standard image High-resolution imageWe next evaluated the synthesized NPs with the hydrothermal autoclaving method. The synthesis of the fabricated Au(III) NPs is confirmed by the gradually changing colors of the solution with the increasing [HAuCl4]3+ salt concentrations. Figure 2 shows that there is a little blue shift (528 nm) in the UV–vis absorption spectra with the increasing salt concentration (1.0 mM), indicating that small size NPs were synthesized. This is in accordance with the study by Angshuman et al [14], where the authors suggested that the main two factors that may hinder and/or affect the diffusion of the growing species to be absorbed or surrounded the growing NPs are: (i) low salt ion concentration (e.g. [HAuCl4]3+) and (ii) polymeric monolayer matrix (e.g. PAAm). If the growth rate of nuclei is limited, the formed NPs are uniformly small in size [14]. Therefore, the increasing of salt concentration (2.5–10 mM) leads to an increase in the absorption intensity and higher wavelength, indicating that the formation of large NPs.
Figure 2. UV–vis spectra of gold nanoparticles synthesized by with hydrothermal autoclaving method, molar [HAuCl4]3+ salt concentration was 0.1–10 mM and polyacrylamide was 1 wt%.
Download figure:
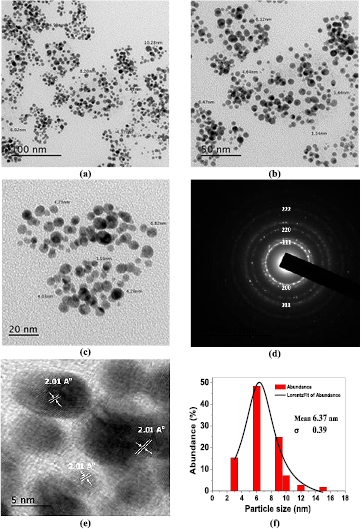
Standard image High-resolution imageThe TEM images demonstrate the formation of AuNPs prepared using both conventional thermal heating and hydrothermal autoclaving techniques. Figure 3 shows typical TEM images at different magnifications, where the morphology and distribution of the Au/PAAm nanocomposite revealed that the synthesized NPs exhibited a spherical shape with a remarkably homogeneous dispersed distribution and an average particle size diameter of 6.37 nm (σ = 1.39), estimated from the corresponding particle size abundance histogram distribution. The equivalent SAED pattern shows polycrystalline circular rings, which can be attributed to the face-centered cubic (fcc) structure of AuNPs. The SAED pattern shows the diffraction pattern with concentric rings that indexed Au(III) NPs from inner to outer, (1 1 1), (2 2 0), 2 2 0, (3 1 1), and (3 1 1) reflections.
Figure 3. TEM micrographs of AuNPs prepared by conventional thermal heating method (a)–(c) at different magnifications, (d) SAED pattern, (e) HRTEM image of a single nanoparticle and (f) particle size abundance histogram.
Download figure:
Standard image High-resolution imageThe HRTEM image of a single NP (figure 3(e)) shows clear lattice fringes with a d-spacing of 2.01 Å. This spacing corresponds to the (1 1 1) spacing for fcc Au [41, 42], confirming the results obtained from UV–vis spectra. Compared to the Au(III) NPs synthesized via the hydrothermal autoclaving route, TEM images of the fabricated NPs were shown in figure 4(a)–(c), with an average particle diameter of 8.19 nm (σ = 0.39); the size of nearly >90% of the synthesized NPs ranged between 3 and 22 nm (figure 4(f)). In addition, the particles were spherical in shape and the SAED pattern displayed bright circular rings of Bragg reflection planes that were attributed to the fcc pattern structure.
Figure 4. TEM micrographs of AuNPs prepared by the hydrothermal autoclaving method (a)–(c) at different magnifications, (d) SAED pattern, (e) HRTEM image of a single nanoparticle and (f) particle size abundance histogram.
Download figure:
Standard image High-resolution image3.1. XRD studies
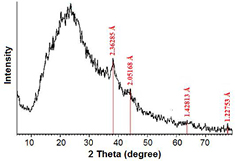
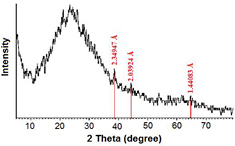
The crystalline nature of the synthesized AuNPs was determined and confirmed using the x-ray diffraction (XRD) technique. Figure 5 shows the XRD spectrum of the fabricated Au/PAAm nanocomposite via conventional thermal heating technique, where the four main characteristic peaks are ascertained to AuNPs at 2θ = 38.17°, 44.37°, 64.55°, and 77.53° that may be correlated to the fcc crystalline structure planes (JCPDS PDF card 04-0784); they can be indexed to the miller indices (dhkl) (1 1 1), (2 0 0), (2 2 0), and (3 1 1) and their interplaner lattice spacing (d) were 2.36 Å, 2.05 Å, 1.42 Å, and 1.22 Å, respectively [43, 44]. Figure 6 represents the XRD spectrum of the synthesized AuNPs with hydrothermal method, where the intensive characteristic peaks of metallic AuNPs were 2θ =38.27°, 44.38°, and 64.65°, corresponding to (1 1 1), (2 0 0), and (2 2 0) crystalline structure of fcc Au-nanocrystal with a lattice parameter of a = 4.09 Å. The XRD spectra showed that the peak corresponding to (1 1 1) was more intense than the other planes, indicating that (1 1 1) is the most proposing orientation of the synthesized AuNPs correlating to the highly crystalline nature of the fabricated NPs with the conventional thermal heating technique compared with the hydrothermal method technique. This result confirmed the data obtained from HRTEM measurements.
Figure 5. XRD spectrum showing the AuNPs/PAAm nanocomposite sample synthesized by conventional thermal heating method.
Download figure:
Standard image High-resolution imageFigure 6. XRD spectrum showing the AuNPs/PAAm nanocomposite sample synthesized by the hydrothermal autoclaving method.
Download figure:
Standard image High-resolution image3.2. The composition and elemental studies
3.2.1. FTIR transmittance analysis
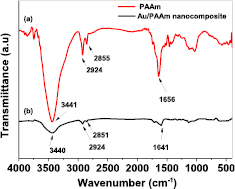
Figure 7 shows the FTIR absorption spectra of Au/PAAm nanocomposites that were prepared by the conventional thermal heating technique. The presence of a certain characteristic peak at 1656 cm−1 was attributed to the C=O stretching (amide I) in the –CONH2 group of PAAm [24, 45]. The band at 1438 cm−1 was associated with the O˗H bending mode. The strong absorption bands at 3441 and 1450 cm−1 were due to the symmetrical and asymmetrical stretching of an N–H group and the bending mode of the –NH2 group in the pure PAAm [15]. The peaks at 2924 and 2855 cm−1 were attributed to the –CH2 and –CH stretching mode. The FT-IR characteristic peaks of the Au/PAAm nanocomposite spectrum show a wavenumber shift from 1656 cm−1 to 1641 cm−1 with a decreasing peak intensity; this may be attributed to the strong interaction between the C=O groups of the –CONH2 group of the polymeric PAAm matrix and Au(III) nanoparticles, leading to the absorption of PAAm molecules on the AuNPs surface [30]. This confirms that a bond is formed between the O atom of the ˗CONH2 group of the PAAm matrix and the Au metallic atoms by the donation of pairs of electrons from the O atom to the metal atoms [46]. In addition, the peaks at 2924, 2855 and 1451 cm−1 were not shifted.
Figure 7. FTIR spectra of (a) pure PAAm and (b) Au/PAAm nanocomposite sample synthesized by the conventional heat treatment method.
Download figure:
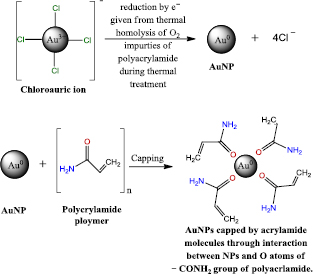
Standard image High-resolution imageFigure 8 shows the FTIR spectra of both pure PAAm and Au/PAAm that were synthesized by the hydrothermal autoclaving method, where the peak at 3418 cm−1 was assigned to the symmetric and asymmetric N–H stretching vibrations of the PAAm polymer. The characteristic band around 1610 cm−1 is due to NH2 bending mode (amide II) [13]. In addition, the dominant peak at 1686 cm−1 was assigned to the carbonyl group (C=O) stretching in the –CONH2 group. The strong absorption band at 1438 cm−1 is attributed to C–N stretching (amide III) [47]. The chemical shift of C=O of the carbonyl group from 1686 to 1680 cm−1 confirms the presence of a coordination bond between the Au atoms and the O atoms of the carbonyl group or the capping by an amino group of polyacrylamide [12]. Moreover, there is no shift of the peak position at 1610 cm−1, reflecting that no interaction was achieved between AuNPs and the N of the N–H group. In addition, the chemical shift from 3418 cm−1 to 3395 may be due to a slight modification of an aN atom of the N–H group due to the heat effect of hydrothermal autoclaving in the presence of high pressure [12]. Scheme 1 shows the proposed fabrication mechanism of the AuNPs through the reduction of [AuCl4]− ion and AuNPs capping by the long alkyl chain of the polymeric PAAm matrix. The [AuCl4]− ion is reduced by e− given from thermal homolysis of O2 impurities of PAAm during the thermal treatment [40] and then capped by the O atoms coordination with (Au0) metallic NPs.
Figure 8. FTIR spectra of (a) pure PAAm and (b) Au/PAAm nanocomposite sample synthesized by hydrothermal autoclaving method.
Download figure:
Standard image High-resolution imageScheme 1. Mechanism of the synthesized AuNPs by the polymeric PAAm matrix.
Download figure:
Standard image High-resolution image3.2.2. EDX studies
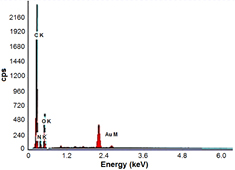
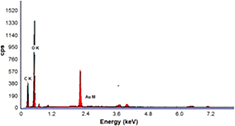
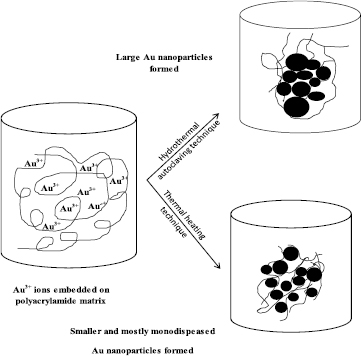
Figures 9 and 10 show the EDX spectra of AuNPs stabilized with PAAm quantitative analysis via both the conventional and autoclaving heating techniques, which confirmed that the elemental composition of the synthesized particle was primarily Au at 2.2 keV with some elemental oxygen and carbon traces [13, 43]. Previous results afforded a clear evidence of AuNPs formation in the polymeric PAAm matrix via the two different techniques; both the techniques yielded small NPs with an average particle size of 6.37 nm and 8.19 nm. Therefore, the suggested mechanism for coating AuNPs with a steric stabilized PAAm polymeric matrix at an elevated temperature (70 °C) for thermal heating technique and (120 °C, 1 atm pressure) hydrothermal autoclaving technique is shown in figure 11.
Figure 9. EDX pattern of Au/PAAm nanocomposite sample synthesized by conventional thermal heating method.
Download figure:
Standard image High-resolution imageFigure 10. EDX pattern of Au/PAAm nanocomposite sample synthesized by hydrothermal autoclaving method.
Download figure:
Standard image High-resolution imageFigure 11. A possible mechanism for the formation of gold nanoparticles in polyacrylamide solution via hydrothermal autoclaving and thermal heating routes.
Download figure:
Standard image High-resolution image3.3. Catalytic activity of AuNPs
The most prevalent and efficient route for 4-nitrophenol reduction is to use NaBH4 as a reductant and a metal nanocatalysts such as AgNPs, AtNPs, PdNPs, NiNPs, and AuNPs. Among the NPs, AuNPs have a remarkable catalytic activity, where the higher catalytic activity of the Au nanocatalyst depends on (i) the size of the NPs (i.e. small NPs size often have a high activity compared to large size one) and (ii) whether the Au nanocatalyst is active even at room temperature. The 4-nitrophenol reduction by NaBH4 is a model catalytic reduction, reported by Langmuir–Hinshelwood; it can be divided into two categories according to the type of nanocatalyst interaction in solution as follows: (i) heterogeneous mechanism where the catalysis takes place on the NP surface and (ii) homogeneous type catalysis occur by leached atoms or ions from NP surface [48].
Two distinct peaks (figure 12(a)) at 317 and 400 nm belong to 4-nitrophenol solution only and the mixture after the addition of NaBH4, respectively. The catalytic activity of AuNPs can be assessed by the reduction and the formation of 4-nitrophenol from 4-nitrophenol in the occurrence of NaBH4. This could be easily detected using UV–vis spectroscopic methods of the reduction reaction. The gradual decrease of the maximum absorption peak at λmax = 400 nm was observed in figures 12(b) and (c) due to the decrease in the formation of 4-nitrophenolate ions from 4-nitrophenol. At the same time, this was accompanied with a gradual growing concomitant appearance of a new peak at 303 nm due to the reduction of 4-nitrophenol to 4-aminophenol in a basic medium at room temperature [49].
Figure 12. UV–vis spectra for (a) 4-nitrophenol before (i) and after (ii) the addition of NaBH4; the reduction of 4-nitrophenol to 4-aminophenol by AuNPs nanocatalyst synthesized with (b) thermal heating route, (c) hydrothermal autoclaving route and (d) plot of ln(At/A0) of AuNPs nanocatalyst towards reduction of 4-nitrophenol.
Download figure:
Standard image High-resolution imageIn figure 12(d), the plots of ln(At/A0) versus time yielded good linear correlations showing that the NaBH4 reduction of 4-nitrophenol catalyzed by AuNPs followed the first-order kinetics. The rate of the equation can be written as kt = ln(A0/At), where k is the rate constant of the first-order kinetics, A0 is the initial concentration of 4-nitrophenol, and At is the concentration at time t.
The observed values of the first-order rate constant (k) and correlation coefficients (R2) for AuNPs prepared via the thermal heating route were 4.19 × 10−3 s−1 and 0.9671, respectively, whereas those for AuNPs synthesized with hydrothermal autoclaving technique were 2.42 × 10−3 s−1 and 0.9556, respectively. The decrease in the catalytic reduction rate (i.e. reduced time) is attributed to the small size of the synthesized NPs via thermal heating technique (approximately 6.37 nm) compared with hydrothermal autoclaving technique (approximately 8.19 nm). The faster catalytic reduction rate of 4-nitrophenol reduction by AuNPs nanocatalysts may be either due to interfacial electron transfer or the rate of electron transfer on the AuNPs surface, which can be influenced by the diffusion of 4-nitrophenol to the metal surface and the diffusion of 4-nitrophenol away from the surface [50, 51].
4. Conclusion
In this study AuNPs were synthesized via two different thermal techniques, conventional heating and autoclaving thermal techniques with an average particle size of approximately 6.37 nm and 8.19 nm and surface plasmon resonance bands at 525 nm and 528 nm, respectively. The synthesized Au/PAAm nanocomposite was characterized XRD, EDX, and FTIR measurements, which revealed that the synthesized AuNPs were strongly embedded and capped with coordination between the carbonyl (–CONH2) group of the polymeric PAAm matrix. The catalytic reduction of 4-nitrophenol by the synthesized NPs was studied. The synthesized nanocomposite showed an effective catalytic activity with a high reaction kinetic rate (4.19 × 10−3 s−1) for smaller NPs synthesized with the convention heating technique than the autoclaving thermal technique (2.42 × 10−3 s−1). Therefore, the fabricated eco-friendly, non-toxic, fast, and cost-effective Au/PAAm nanocomposite is highly recommended as a nanocatalyst for the removal of different environmental chemical pollutants.