Abstract
Copper-doped zinc oxide nanoparticles were successfully synthesized by grinding copper acetate and zinc acetate powder with different starting molar ratios in combined with sodium hydroxide. The effect of initial copper and zinc molar ratios on the product samples was investigated and discussed. Relevant ligand coordination type of reactant acetate salt precursors and product samples were investigated by Fourier transform infrared spectroscopy (FTIR). The particle shapes and surface morphologies were characterized by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). Phase structures of prepared samples were studied by x-ray powder diffraction (XRD) and x-ray absorption near-edge spectroscopy (XANES) was applied to investigate the local structure of Cu and Zn environment atoms. The results demonstrate that the, particle size of as-synthesized products affected by copper concentration in the precursor trend to gradually decreases from nanorod shape with diameter around 50–100 nm to irregular particle structure around 5 nm associated with an increase in the concentration of copper in precursor. Moreover, it is noticed that shape and morphology of the products are strongly dependent on Cu:Zn ratios during the synthesis. Nanocrystallines Cu-doped ZnO by the substitution in Zn site with a high crystallization degree of hexagonal wurtzite structure were obtained. This synthesis technique is suggested as a potential effective technique for preparing copper zinc oxide nanoparticles with various atomic ratio in wide range of applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Cu-doped zinc oxide nanoparticles or copper–zinc–oxide nanocomposites have been intensively studied because it has considerable potential for applying to wide range of applications, such as optical coatings, light-emitting diodes, laser diodes and catalysts. Recently, the unique properties demonstrated in Cu-doped zinc oxide nanoparticles have gained great interest for developing a wide range of advanced applications including field effect transistors [1, 2], field emission arrays, ultraviolet lasers, light emitting diode [3], sensors, biosensors [4, 5], catalyst [6, 7], energy storage and solar cell [8]. The advanced functional properties of nanostructure materials are closely related to several factors such as high surface mass ratio, selective control surface terminal, different local structure from bulk, magnetic property and also electronic structure. For example, in Cu-doped zinc oxide, its magnetic property and band gap can be controlled by either changing its local structure or oxygen and/or Zn vacancies concentration by Cu1+ and/or Cu2+ substitution interstitial of ZnO. All those factors can be controlled by modifying the nanostructures and most of obtained nanostructure materials obey the specific synthesis method and conditions.
Various techniques have been used to synthesize Cu-doped zinc oxide nanoparticles and copper–zinc–oxide nanocomposites by many researches such as hydrothermal, co-precipitation, sono-chemical and ball milling. Synthesis methods based on solid state reaction exhibit considerable interest to apply for synthesizing nanoparticles or nanocomposites since the reaction only happens at interface of precursor, which is anion and cation and/or intermediate has limitation to move. CuO nanopowder with the particle diameter of 20 nm has been synthesised by one step solid state reaction using CuCl2·2H2O with NaOH at room temperature [9]. ZnO microflakes has been prepared by solid state reaction using bis(acetylacetonato)zinc(II), Zn(acac)2 as precursor with dodecyl sulfate (SDS) and NaOH as reactant [10]. The nanoparticles are successful synthesized by the solid state reaction method using the reagents of ZnSO4·7H2O with NaOH [11]. Cu-doped zinc oxide powders has been prepared by solid state method the range of 0.25–15 mole% Cu by mixing and homogenising of Cu with ZnO at high temperatures [12]. Vidyasagar et al [13] employed solid state reaction to synthesize Cu-doped zinc oxide nanoparticles by using acid salts of ZnCl2, CuCl2 and NaOH reagents with polyethylene glycol 400. Wang et al [14] used solid Cu(NO3)2·3H2O and Zn(NO3)2 with Na2CO3 to prepare Cu-doped zinc oxide nanoparticles through solid state reaction. However, these solid state synthesis methods operated at high temperature condition and obtained large particle size of Cu-doped ZnO such as pellets particle [1, 12] and undoped ZnO microflakes [10]. The solid state synthesis of nanoparticle were achieved for undoped CuO [9] and ZnO [11] while Cu-doped ZnO nanoparticle was obtained by assistance of polyethylene glycol surfactant reagent [13]. However, annealing process of washed product over 300 °C is necessary to ensure the removal of all impurities that would affect to the crystal growth and size of synthesized particles [13]. Herein, this work, we develop the solid state method by improving the high homogeneity of copper and zinc in acetate salt and preparing the starting acetate salt precursor from CuO and ZnO at different ratios to obtain high homogeneity of copper and zinc in acetate salt. The solid state reaction is conducted in air at room temperature without either surfactant or calcination to result the small particle and the synthesis time is within 15 min. A numerous advances of this synthesis technique are reported, the local environment of Cu and Zn central atom are further investigated by XANES experiment.
2. Experimental
2.1. Material
The starting ZnO, CuO and acetic acid were of industrial grade and used as received without further purification. All the standard materials as referent used in our experiments were of analytical grade, zinc acetate purchased from Ajax Finechem Pty Ltd, copper acetate, zinc oxide and copper oxide ⩾99.9% were procured from Sigma Aldrich.
2.2. Synthesis of copper zinc acetate precursors
The copper zinc acetate precursors were prepared by mixing CuO with ZnO for different mole ratio. The obtained mixtures of CuO/ZnO powder were mixed with glacial acetic acid in the ratio of 1:2 by mole followed by DI water for equal volume. The mixture solution was boiled and stirred by magnetic stirrer and heated by hotplate at 90 °C until clear solution was obtained. The solution was maintained in hot air oven at 90 °C for 24 h and then kept in room temperature for crystallization. Sedimented acetate salt was collected and washed with cold DI water then dried in oven at 80 °C for 24 h. The prepared copper zinc acetate salts from the mole% of Cu/(Cu + Zn) at 0.01, 0.10, 1.0, 10.0 and 50.0 mixture powders are denoted as CZOAc-Cu0.01, CZOAc-Cu0.10, CZOAc-Cu1.0, CZOAc-Cu10 and CZOAc-Cu50, respectively.
2.3. Synthesis of copper zinc oxide nanoparticles
Copper zinc oxide nanoparticles were prepared by mixing the copper zinc acetate salt powder with sodium hydroxide powder for 1:2 by mole and grinding in glass mortar for 15 min. The mixed product was then washed with distilled water for 5 times and dried in oven at 80 °C for 24 h. The copper zinc oxide nanoparticles prepared from CZOAc-Cu0.01, CZOAc-Cu0.10, CZOAc-Cu1.0, CZOAc-Cu10 and CZOAc-Cu50 are assigned as CZO-Cu0.01, CZO-Cu0.10, CZO-Cu1.0, CZO-Cu10 and CZO-Cu50, respectively.
2.4. Characterization
The corresponding ligand coordination and functional groups of powders were analyzed by FTIR IRTracer-100 Shimadzu in the range of 400–4000 cm−1. The XRD of products was recorded by a XRD (PANalytical X'Pe°Pro) at 40 kV, 30 mA using Cu-Kα radiation. FESEM images were obtained on FE-SEM model Hitachi-S4700 and higher resolution was obtained by TEM image. X-ray absorption spectroscopic (XAS) measurements were performed at the Zn and Cu K-edge were performed on transmission mode using Ge(2 2 0) double-crystal monochromator synchrotron radiation at beam line 8 station of the Synchrotron Light Research Institute (SLRI), Nakhon Ratchasima, Thailand.
3. Results
3.1. Copper zinc acetate
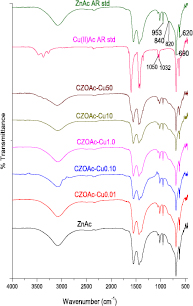
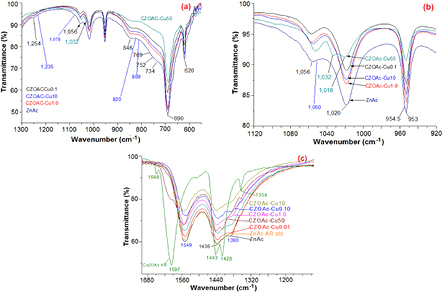
Fourier transform infrared (FTIR) spectroscopy was performed to study the prepared copper zinc acetate salt and the corresponding spectra are shown in figure 1. The band at 620 cm−1 can be attributed to the acetate anion twisting mode and the band at 690 cm−1 can be assign to the acetate anion scissoring as shown in figure 2(a). Meanwhile, the band at 809 cm−1 can be attributed to vibration mode of CH2, the band at 820 cm−1 can be attributed to the C–C stretching vibration mode and the band at 846 cm−1 can be attributed to the vibration mode of C–C skeletal. The band at 1235 cm−1 can attributed to the (C–O) stretching vibrations mode and the band positioned at 1056 and 1032 cm−1 can be attributed to vibration of C–CH3 framework. Moreover, the band at 1050 cm−1 can be attributed to the C–O–C stretching vibration mode and the band at 1078 cm−1 can be attributed to the CH3 asymmetric bend. All of the bands represented as an enlargement in figure 2(b) are the characteristics of propionic acid which is in a form of acetic [15–18]. The band at 1018, 1032 cm−1 and C–O–C symmetric stretch vibration at 1050 cm−1 are the finger print of copper acetate while the strong band at 1020 cm−1 and the weak peak shoulder at 1056 cm−1 of C–CH3 framework is originated from zinc acetate.
Figure 1. FTIR spectra of the prepared copper zinc acetate salt and standard copper acetate and zinc acetate.
Download figure:
Standard image High-resolution imageFigure 2. Enlargement of FTIR spectra of the prepared copper zinc acetate samples: acetate anion twisting and scissoring (a), acetate salt finger print (b), carboxyl group bridging coordinated in inorganic salt (c).
Download figure:
Standard image High-resolution imageThe wavenumbers at 1550 and 1390 cm−1 in figure 2(c) typically assign to the antisymmetric and symmetric stretching vibration mode of COO−, respectively. The carboxyl group in inorganic salt are coordinated with metal ion coordinating to divalent metal cations that can be found in four modes, the vibration mode energy of carboxyl group depending on coordination mode and the type of metal ion. The bonding energy for zinc cation with acetate anion in zinc acetate increases in the order unidentate > bridging > bidentate [18–23]. Two dominate FTIR bands of COO− symmetric stretching vibration observed in samples at 1390 and 1438 cm−1 can be used to determine coordination type of prepared copper zinc acetate samples. The different in wavenumber between antisymmetric and symmetric vibration modes equals to 160 and 112 cm−1 indicating that the coordination type of acetate in prepared samples are close to the composition of two type, unidentate and bridging-type. CZOAc-Cu50 shows different band structure by pattern shift close to copper acetate standard due to high mole ratio of copper in copper acetate. In addition, the observable bands situated at 3500 to 2800 cm−1 assign to O–H stretching vibration mode from intermolecular hydrogen bonding in acetate salt and/or water adsorption and the band at 3350 cm−1 is associated to O–H stretching vibration mode of water. Meanwhile, the bands at 2970 to 2850 cm−1, 2990 cm−1 and 2420 cm−1 are corresponded to C–H stretching vibration mode of methyl groups. The characteristic band of copper acetate salt at 1597, 1443, 1420, 1354, 1032 and 1018 were present dominated in CZOAc-Cu50 sample may be due to limit to homogeneous and lead to zinc acetate tend to form in bridging ligand coordination.
The results from FTIR clearly indicate that copper zinc acetates were obtained without the signal of impurity or contaminant or by product such as sodium acetate. The appeared color of obtained copper zinc acetate salt powders is in white to dark blue depending on copper concentration as observed in figure 3.
Figure 3. Photograph of prepared copper zinc acetate sample, the colour appear white to blue to dark blue by order of copper concentrations of 0.01, 0.10, 1.0, 10.0 and 50.0 mol%.
Download figure:
Standard image High-resolution image3.2. Copper zinc oxide nanoparticles
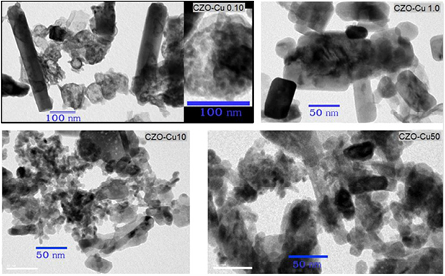
FESEM images in figure 4 show morphologies and structures of the prepared copper zinc oxide nanoparticles prepared from the different mole ratios of copper to zinc precursor. There is remarkable morphology change in shape and particle size. The appearance of morphological shape and particle size may be attributed to the incorporation of Cu2+ to the Zn2+ sites during the crystallization. The high zinc molar ratio of CZO-Cu0.10 sample consists of small irregular particle aggregates with a rod-like shaped structure with 500 nm long and diameter of about 50–100 nm. Meanwhile, CZO-Cu1.0 sample shows nodular spherical like shape in the particle size around 30–50 nm with more homogeneous size distribution. CZO-Cu10 exhibits irregular particle shape and noticeable aggregation with particle size ranging from 20–100 nm and CZO-Cu50 sample shows non-uniform particle shape and size with particle size ranging from 5–500 nm.
Figure 4. FESEM images of the prepared copper zinc oxide nanoparticles CZO-Cu0.10, CZO-Cu1.0, CZO-Cu10 and CZO-Cu50.
Download figure:
Standard image High-resolution imageThe transmission electron microscopic (TEM) analysis was carried out for achieving high accuracy of actual particle size and shape pattern. The typical morphologies from TEM image are shown in figure 5, revealing that CZO-Cu0.1 sample consists of the nanorod shape with diameter range of 50–100 nm and 200–500 nm in length with aggregation of small spherical primary particles whose size is around 5 nm as seen in the inset of figure 5 (CZO-Cu0.1). TEM image accompanying FESEM image of CZO-Cu1.0 sample indicates nearly hexagonal short nanorod shape with diameter of 20–50 nm. Meanwhile, CZO-Cu10 samples show the composition of small nodular particle with 5–10 nm diameters. The CZO-Cu50 reveals the assembly of irregular structure with particle size ranging from 5 to 50 nm. It is clearly seen from the FESEM and TEM micrographs that the morphology shape and size of copper doped zinc oxide sample was affected by the amount of Cu:Zn mole ratio in acetate salt precursor. As the concentration of copper in acetate salt precursor since 1.0 mole% and lower, the nanorods were appeared and notable exhibiting better nanorod shape at 0.1 mole% Cu of precursor.
Figure 5. TEM image of the prepared copper zinc oxide nanoparticles CZO-Cu0.10, CZO-Cu1.0, CZO-Cu10 and CZO-Cu50.
Download figure:
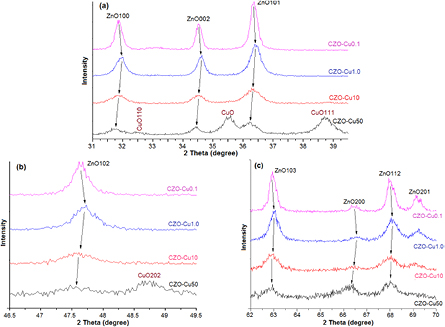
Standard image High-resolution imageFigure 6 shows the XRD peaks of all synthesized copper zinc oxide nanoparticles obtained from different zinc and copper mole ratio of starting precursor, demonstrating the crystalline character of the copper doped zinc oxide nanocrystals. The diffraction peaks at 2θ around 31.9°, 34.5°, 36.5° of plane; 47.7° are assigned to hexagonal zinccite ZnO 1 0 0, 0 0 2, 1 0 1 (figure 6(a)), 2θ = 47.7° is assigned to hexagonal zinccite ZnO 102 of plane (figure 6(b)); 2θ at 63.0°, 66.5°, 68.0° and 69.2° are assigned to hexagonal zinccite ZnO 1 0 3, 2 0 0, 1 1 2 and 2 0 1 of plane (figure 6(c)), respectively. Moreover, the peaks at 2θ positioned at 35.7°, 38.9°, 49.2°, 58.5° and 62.0° correspond to the CuO −1 1 1, 1 1 1, −2 0 2, 2 0 2 and −1 1 3 plane, respectively. The diffraction pattern for CZO-Cu0.1 and CZO-Cu1.0 samples can be indexed to the ZnO hexagonal nanorods with the wurtzite structure. The typical FWHM of the XRD peak tends to widen while the peak intensity decreases with increasing copper content in acetate salt precursor. This behaviour attributes to the decreasing crystallite size with increasing Cu dopant. The most strong and narrow peak is ZnO (1 0 1) with the other peaks (i.e. ZnO (0 0 2) parallel to the c-axis) show the decrease in peaks intensity and the peak broadening suggesting that the Cu-doped ZnO crystal prefers to grow along c-axis by this synthesis method. The secondary phase CuO peaks pattern was found in CZO-Cu50 sample and the weak broadening ZnO peaks indicating the smaller crystalline size obtained in high at copper 50 mole% ratio. ZnO peak additionally exhibits slight shift implying that a small part of CuO may incorporate into the ZnO wurtzite lattice. The secondary phase of CuO diffraction peaks indicated that formation of CuO–ZnO nanocomposite and the doping limit for Cu-doped ZnO sample of our synthesis method is attained with copper content lower than 50 mole% ratio precursor. The XRD peaks of Cu-doped ZnO samples also shift slightly toward higher Bragg's angle indicating an increase of the lattice constant. The slight change in the lattice parameters and crystallite size suggest that the substitution of Cu ion into the Zn site could be occurred [24, 25]. In addition, the other phases such as NaAc by product or Zn(OH)2 intermediate product were not found in XRD patterns indicating that the final product is in high purity.
Figure 6. The results of XRD patterns of copper doped zinc oxide nanoparticles samples synthesized with different Cu:Zn mole ratios of acetate salt precursors induced peak shift.
Download figure:
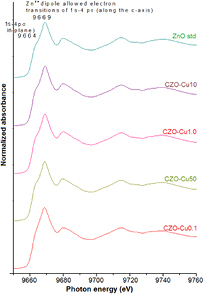
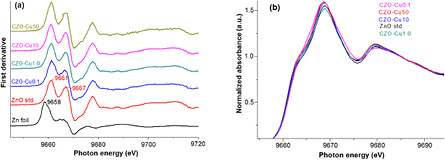
Standard image High-resolution imageFigure 7 illustrates normalized and background-subtracted Zn K-edge XANE spectra for copper zinc oxide nanoparticles obtained from different copper mole ratio of starting precursor comparing with ZnO standard hexagonal and corresponding 1st derivative (figure 8(a)). The Zn K-edge absorption energy for all samples is at 9661 eV that is the same edge position corresponding to Zn2+ oxidation state of zinc atom. The main peak located at 9669 eV along with a shoulder at 9664 eV corresponds to the two peaks of the first derivatives at 9667 eV and 9661 eV can be assigned to the Zn2+ dipole allowed 1s–4pπ transitions along the c-axis and 1s–4pσ in-plane, respectively [26–28]. All Zn K-edge XANE spectra display similar features compared to standard ZnO. The edge energy position and pattern is almost identical along with a slight oscillation change, implying that the environment atom of zinc atom has exactly the same structure to the hexagonal wurtzite ZnO structure in all copper concentration in the matrix. The slight oscillation change (figure 8(b)) is likely the result of a change in nanosized and/or amorphous character of sample particle and/or the reduction of long-range order leading by Cu ions occupying the Zn site.
Figure 7. Normalized Zn K-edge XANE spectra of copper zinc oxide nanoparticles comparing with ZnO standard zinccite hexagonal.
Download figure:
Standard image High-resolution imageFigure 8. The corresponding 1st derivative (a) and enlarge (b) of oscillation pattern.
Download figure:
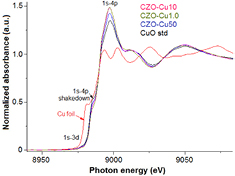
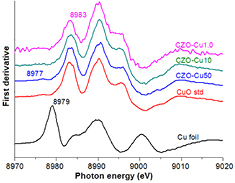
Standard image High-resolution imageFigure 9 shows the normalized Cu K-edge XANE spectra and along with the 1st derivative in figure 10. For comparison, the data of reference bulk CuO and Cu metal are also presented. The Cu pre K-edge located at 8977 eV which is pronounced in the first derivative, this feature of 1s → 3d and 1s to dipole-allowed to p–d hybridization orbital, more clearly in the first derivative patterns in figure 10. All of the samples and standard CuO shows the same Cu K-edge absorption edge at 8983 eV which is assigned to the allowed 1s → 4p transition corresponding to Cu2+ oxidation number in bulk CuO [29–31]. Typical XRD peak shift can be explain lattice strain and stress due to defect or vacancies and/or lattice stress and strain may induce by substitution of Cu ion into the ZnO lattice. However, Cu1+ and Cu+ ions are also substituting the Zn2+ sites and XRD peak change. In this work the XRD peak shift inconsistently by Cu concentration precursor could be assumed Cu valence state of +1 or/and +2 in the Cu-doped ZnO samples possibly, due to the substitution of Cu2+ ion into the Zn site lead to lattice parameter smaller and when Cu1+ ions substituted Zn2+ ions induce the lattice constant increased. The result XANES spectra of all Cu-doped ZnO samples are identical and clearly related to the CuO reference in terms of edge position and white line intensity except for tiny differences in the profile of the rising edge and the resonance peaks after the edge, these result indicated the exactly only Cu2+ ion substitution for Zn2+ ions without changing ZnO wurtzite structure in all samples and without Cu, and Cu2O phases present in the samples.
Figure 9. Normalized Cu K-edge XANE spectra of copper zinc oxide nanoparticles samples.
Download figure:
Standard image High-resolution imageFigure 10. The corresponding 1st derivative and enlarge of pre K-edge pattern.
Download figure:
Standard image High-resolution imageThese results of Cu atoms occupying the Zn sites by substitutionally in the hexagonal ZnO host lattice, due to the solid contact and strong reaction in the solid state reaction can control the diffuse or migration of intermediate during reaction within a short time led the fast crystal core forming controlling crystal growth formation in a nanoparticle. The strong reaction induced high crystallinity in nanoparticle without calcinations necessary. Due to local structure of CuO close to ZnO, the coordination number and the Zn–O bond property almost same Cu–O bond therefore help to the high Cu concentration in well hexagonal wurtzite crystalline of Cu-doped ZnO nanoparticle can be obtain by this synthesis technique. By this synthesis technique can develop to preparation of Cu-doped ZnO nanorod structure which is one of the major challenges to reduced diameter of metal-doped ZnO nanorods as very low defect with high crystal perfection and without using surfactant or template.
4. Conclusions
Highly homogeneous copper zinc acetate with different copper mole ratios were successfully prepared from mixture of CuO and ZnO with different ratios. The copper zinc acetate salts show highly homogeneous without undesired by product obtained until ratio of Cu at 50 %mole and show excellent for use as precursor to synthesis of Cu-doped ZnO nanoparticles in solid state reaction process. The Cu-doped ZnO nanoparticles show high crystallinity of hexagonal wurtzite and pour were obtained with Cu2+ substitute in Zn2+. The second phase of CuO was found in the case of Cu 50 %mole precursor corresponding to inhomogeneous of the prepared acetate salt precursor. The morphology and particle size of obtained Cu-doped ZnO nanoparticles depend on Cu concentration in the acetate salt precursor and trend to size decrease with increase of Cu concentration in the acetate salt precursor. The Cu-doped ZnO nanoparticles show favour growth along c-axis. This study present potential effective technique for prepare copper zinc oxide nanoparticles.
Acknowledgment
The author would like to appreciate beamline 8 (BL8) Synchrotron Light Research Institute (Public Organization) for technical and resource supporting this work.
Footnotes
- *
Contribution at the 4th Southeast Asia Conference on Thermoelectrics 2016 (SACT 2016), 15–18 December 2016, Da Nang City, Vietnam.