Abstract
In the present work, nano-sized TiO2 polymorphs (anatase, brookite, and rutile) were synthesized via hydrothermal treatment of an amorphous titania. Three polymorphs were characterized by XRD, Raman spectroscopy, SEM, UV–Vis DRS, and N2-sorption measurements. The photocatalytic degradation experiments were performed with low catalyst concentration, high organic loading under a 60 W UV–Vis solarium lamp irradiation. The photocatalytic degradation was monitored by UV–Vis spectroscopy and TOC measurements. Cinnamic acid, ibuprofen, phenol, diatrizoic acid and the dyes rhodamine B and rose bengal were used as model pollutants. The formation of intermediates was studied by ESI-TOF-MS measurements. The presence of active species was checked by quenching the activity by addition of scavengers. The photocatalytic activity decreased in the order: anatase ⩾ brookite > rutile, with growing recalcitrance of organic compounds. The differences in the activity are more pronounced in the degree of mineralization. The valence band holes and superoxide radicals were the major active species in the photocatalytic treatment with anatase and brookite, whereas hydroxyl radicals and superoxide radicals contributed mainly in the treatment with rutile explaining the lower activity of rutile. The complementary use of UV–Vis spectroscopy and TOC measurements was required to obtain a comprehensive realistic assessment on the photocatalytic performance of catalyst.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Pharmaceuticals, x-ray contrast agents, phenolic compounds and dyes have been considered as recalcitrant pollutants presented in water and wastewater [1–5]. Their compositions, strength as well as the degree of occurrence depend on the source. Domestic and industrial are reported as the popular sources of wastewater. They cannot be removed completely due to their trace with low concentration in water and resistance traditional treatment methods as biological degradation, adsorption, ozonation [6–9]. Heterogeneous photocatalysis, advanced oxidation processes (AOPs), have been realized as a particularly efficient process to achieve the complete degradation and mineralization of hazardous organic pollutants. Under irradiation (UV or visible light) catalysts can form highly active species (holes, electrons, hydroxyl radicals, superoxide radicals) to decompose and mineralize organics into CO2 as final oxidation product [10–12]. Additionally, catalysts in solid form can be easily separated from the treated aqueous solution to reuse [13]. Titanium dioxide (TiO2) has a high chemical stability, high oxidizing ability, and low cost [14–18]. Therefore, it is widely used as a semiconductor photocatalyst for various photocatalytic reactions as hydrogen production or degradation of pollutants in aqueous solutions. Many researchers investigated the photocatalytic behaviour of TiO2 on the different titania polymorphs including anatase and rutile mostly, or on the biphasic [19–25] and triphasic [20, 21, 25–28] mixtures, and less with brookite [29–31] to improve the photocatalytic activity and enhance the photocatalytic degradation of pollutants effectively. However, most studies deal with the photocatalytic decolourization of dyes [19, 32–35]. The results were obtained at much high times excess of catalyst by mass compared to the dyes (20–100 times) and high irradiation power emitted from the Xe lamp or Hg lamp (150–300 W). These conditions are far from the requirements for the photocatalytic degradation of aromatic units mostly present in antibiotic pharmaceuticals. Also, the degree of mineralization of organics has not been studied and reported so far. Among three titania polymorphs, the synthesis and study on the photocatalytic activity of brookite, a promising photocatalyst, has been less studied. It is believed that the problem is not due to the activity but rather the metastable state.
Hence, the objective of this study is to prepare three titania polymorphs by hydrothermal synthesis at elevated temperatures in acidic media and investigate in detail their photocatalytic performances under simulated solar light equivalent UV. For this purpose, a series of photocatalytic degradation experiments and analysis by UV–Vis spectroscopy (the cleavage of aromatic ring), TOC (change in total organic carbon), and ESI-TOF-MS (the formation of reaction intermediates) were performed, which also allow to give a comprehensive realistic assessment on the photocatalytic degradation of aromatics. Cinnamic acid, ibuprofen, phenol, diatrizoic acid and dyes rose bengal, rhodamine B were used as model pollutants. Finally, the selective scavenger experiments could give a detail view about the differences in the activity between three titania polymorphs by the contribution of active species.
2. Experimental
2.1. Materials
All chemicals were of analytical grade and used without further purification: titanium (IV) i-propoxide (TTIP, Merck, 98%), hydrochloric acid (HCl, Chemsolute, 35–38%), glacial acetic acid (CH3COOH, Chemsolute, 99.7%), i-propanol (C3H8O, Sigma-Aldrich, >99%), titania P25 (TiO2, Degussa, 99.5%), ibuprofen sodium salt (C13H17O2Na, Sigma-Aldrich, 98%), cinnamic acid (C9H8O2, Reachim, 99%), phenol (C6H6O, Sigma-Aldrich, 99%), diatrizoic acid (C11H9I3N2O4, Sigma-Aldrich, 98%), rhodamine B (C28H31ClN2O3, Sigma-Aldrich, ⩾95%), rose bengal sodium salt (C20H2Cl4I4Na2O5, Sigma-Aldrich, ⩾85%), ethylenediaminetetraacetic acid (C10H16N2O8, Sigma-Aldrich, 99%), tert-butanol (C4H10O, Sigma-Aldrich, 99%), 1,4-benzoquinone (C6H4O2, Sigma-Aldrich, ⩾98%).
2.2. Synthesis of titania polymorphs (anatase, brookite, rutile)
Anatase, brookite and rutile were prepared according to our previous report [36]. Detailed procedure for the synthesis is as follows
2.2.1. Sol-gel synthesis of amorphous titania precursor.
20 ml of titanium (IV) i-propoxide was dissolved first in 105 ml of i-propanol under vigorous stirring. The mixture was cooled down in an ice bath and maintained at 0 °C for further step. The second solution containing 105 ml of i-propanol and 1 ml of water was prepared at room temperature (RT) and then added slowly into the above mixture. The latter mixture solution was further stirred at RT for 24 h. Thereafter, the white solid was removed from the suspension by centrifugation. The remaining solution was again diluted with 1000 ml of water, then further stirred at RT for 24 h, and finally separated by centrifugation. The final obtained solid was washed with water and ethanol and then dried in vacuum at 60 °C.
2.2.2. Hydrothermal synthesis of titania polymorphs.
1.0 g of the obtained amorphous titania powder was given into the 120 ml teflon cup and then a concentrated aqueous acid solution was added to achieve a 0.3 M solution. The latter suspension was stirred at RT for 30 min. The teflon cup was then placed inside a stainless steel-lined autoclave and heated at elevated temperatures. The hydrothermal treatment in 1.5 M acetic acid (CH3COOH) aqueous solution at 200 °C was carried out to form anatase. Under the same temperature, the use of 4.0 M hydrochloric acid (HCl) yields the rutile. When HCl concentration was changed to 3.0 M and the reaction temperature was set at 175 °C, brookite was formed. After hydrothermal heating for 7 h, the autoclave was cooled to RT naturally, and the obtained white precipitates were decanted, washed thoroughly with distilled water and ethanol. Finally, they were dried at 60 °C overnight. All products were grinded in a porcelain mortar with a pestle to get the fine white powder.
2.3. Characterization
The morphology of synthesized titania polymorphs were characterized by scanning electron microscopy (SEM) using an S4800 field emission scanning electron microscope (FE-SEM, Hitachi, Japan) at an accelerating voltage of 5 kV. The x-ray diffraction (XRD) patterns were recorded on a STADI-P x-ray diffractometer (STOE) using monochromatic CuKα radiation (λ = 1.5406 Å). Diffuse reflectance UV–Vis spectra were measured at RT using a Cary 5000 spectrometer (Varian) equipped with a diffuse reflectance accessory (praying mantis, Harrick) and converted into the Kubelka-Munk function F(R). By plotting (F(R)·hν)1/2 versus (hν) and (F(R)·hν)2 versus (hν) for indirect and direct band gaps, respectively, Tauc plots were obtained. hν is the photon energy and F(R) is the absorption coefficient. The band gaps (Eg) were determined by extrapolating the straight line of the plots at the point (F(R)·hν)1/2 = 0 and (F(R)·hν)2 = 0 on hν-axis [29, 37, 38]. N2 adsorption-desorption were measured at 77 K on a Thermo Sorptomatic 1990 instrument. The specific surface area was determined by the Brunauer–Emmett–Teller (BET) method using the relative pressure (p/p0) range of 0.05–0.35. The UV–Vis absorption spectra of aqueous reaction solutions were measured using a Lambda 19 UV–Vis spectrometer (Perkin Elmer). The mineralization of organics was measured by a TOC analyser (TOC-L CSH/CSN, Shimadzu). The formation of intermediates was studied by electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) using an electrospray ionization mass spectrometer HPLC System 1200/ESI-TOF-MS 6210 (Agilent). An aqueous solution containing 10 vol.% of methanol (HPLC, gradient grade, ⩾ 99.8%) and 0.1 vol.% of formic acid (HCOOH) was used as mobile phase. The flow rate was 1.0 ml min−1. The mass-to-charge (m/z) was scanned in the mass range of m/z of 0–1000.
2.4. Photocatalysis
The photocatalytic performances of TiO2 samples were evaluated in the degradation of cinnamic acid (CA), ibuprofen (IBP), phenol (Ph), diatrizoic acid (DA) and the dyes rhodamine B (RhB) and rose bengal (RB) as model organic pollutants under UV–Vis irradiation using batch-conditions. In each experiment, a glass beaker containing 10 mg of the photocatalyst and 250 ml of an aqueous 10 ppm organic compound solution was used. The reaction mixture was magnetically stirred in dark at RT for 30 min to reach the adsorption equilibrium. The UV–Vis solarium lamps (15 W, Philips) with a total power of 60 W were used as light source. These lamps simulate the UV part of sunlight (by light energy distribution and intensity) in the range of 370–400 nm. The distance between the applied lamps and the surface of pollutant solution was 15 cm. After certain intervals, 5 ml aliquots were taken from the reaction mixture by a syringe and separated from the catalyst by a 0.45 µm PTFE filter. The abatement of aromatic rings and decolourization of dyes were determined by the change in the intensity of the corresponding absorption bands of studied organic compounds of following equation [39]
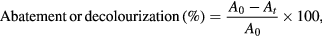
where A0 and At are the initial absorbance and the absorbance after various time intervals of UV–Vis irradiation (t), respectively. All data were obtained at RT.
The presence of active species was studied by scavenger experiments. In which, 1.46 mg of ethylenediaminetetraacetic acid (EDTA) acted as hole (h+) scavengers, 0.1 ml of tert-butanol (t-BuOH) acted as hydroxyl (•OH) radical scavengers, and 2.704 mg of 1,4-benzoquinone (1,4-BQ) acted as superoxide ( ) radical scavengers were used.
) radical scavengers were used.
3. Results and discussion
3.1. Characterization
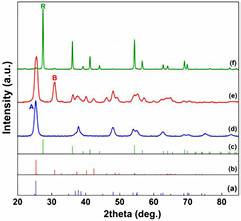
The crystalline structure and crystallinity properties of synthesized titania polymorphs were studied by powder XRD (figure 1). All XRD patterns show that the intensity and diffraction angle of reflections agree with the theoretical diffraction pattern from the JCPDS databases (curves (a)–(c) in figure 1). This indicates that the individual titania phases were readily formed under the certain hydrothermal synthesis conditions. They were highly crystallized. The width of diffraction reflections in the patterns of anatase (curve (d) in figure 1) and brookite (curve (e) in figure 1) are broad, which indicates the formation of nanoparticles consisting of small crystallites. In contrast, they are much narrower in the pattern of rutile showing the higher degree of crystallinity and larger crystallite size. The formation of pure brookite was confirmed by the presence of the intense diffraction reflection at 2θ = 30.8° (1 2 1) and the absence of the typical diffraction reflection of anatase at 2θ = 62.3° [28, 30, 36]. By means of Scherrer equation, anatase and brookite have small crystallite sizes of 10–12 nm (table 1), whereas crystallite size of rutile is relatively larger, of 98 nm.
Table 1. Characteristics of synthesized titania polymorphs: phase composition, specific surface area (SBET), band gap energy (Eg), absorption wavelength (λabs), crystallite size (DXRD) determined by XRD, particle size D (W-width, L-length) determined by SEM; SBET and density of TiO2 [36].
| Sample | Phase content (%) | SBET (m2 g−1) | Eg (eV) | λabs (nm) | Particle size (nm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | R | XRD |
SBET | SEM | ||||
| Anatase | 100 | 132 | 3.25 |
387 | 10 | 11 | 10 | ||
| Brookite | 100 | 116 | 3.25 |
387 | 12 | 12 | 10 | ||
| Rutile | 100 | 12 | 3.34 |
413 | 98 | 117 | W: 50–100, | ||
| L: 300–500 | |||||||||
aCalculated for the indirect and direct band gaps, respectively. bCrystallite size.
Figure 1. XRD patterns of synthesized titania polymorphs. JCPDS databases (a) 96-900-9087 for anatase (A), (b) 96-900-9088 for brookite (B), and (c) 96-900-9084 for rutile (R).
Download figure:
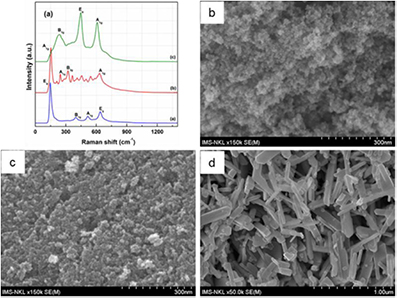
Standard image High-resolution imageThe structure and purity (pure phase) of titania samples were further confirmed by Raman spectroscopy. Figure 2(a) shows the Raman spectra of distinctive titania polymorphs anatase, brookite, and rutile, respectively. The typical Raman bands of pure anatase were observed at 147, 398, 516, and 638 cm–1 which are assigned to the Eg, B1g, A1g and Eg modes, respectively [26]. The Raman spectrum of brookite samples is more complex. It shows an intense Raman band at 155 cm–1 which is ascribed to the vibrational mode A1g. The weaker Raman bands were observed in the range of 200–700 cm–1. Brookite is very well distinguished from anatase by the absence of Raman band of anatase at 516 cm–1 in the Raman spectrum of brookite [30, 40]. The distinctive Raman bands for pure rutile appeared at 245, 442, and 607 cm–1 are ascribed to B1g, Eg and A1g modes [41, 42]. The Raman results were further confirmed that all synthesized titania polymorphs are of high quality and of phase-pure, which are consistent with the XRD results.
Figure 2. (a) Raman spectra and SEM images of (b) anatase, (c) brookite, and (d) rutile.
Download figure:
Standard image High-resolution imageThe morphology and particle size of titania polymorphs samples were studied by SEM (figure 2 and table 1). This indicates that the hydrothermal treatment led to the formation of well-developed and randomly distributed nanoparticles. Pure anatase and brookite were formed in the spherical particles with average size of 10 nm (figures 2(b) and (c)). This value is in good agreement with the XRD results of crystallite sizes determined by Scherrer equation which assumes spherical and cubic shapes mostly. In contrast, rutile crystallized in the shape of rod-like nanocrystals of 50–100 nm in width and 300–500 nm in length (figure 2(d)).
3.2. Photocatalysis
3.2.1. Photocatalytic activity.
The photocatalytic performances of three titania polymorphs were evaluated first in the degradation of cinnamic acid (CA), ibuprofen (IBP), phenol (Ph), diatrizoic acid (DA) with a general recalcitrant order as follows: CA < IBP < Ph < DA. Figure 3 shows the photocatalytic abatement obtained after 1 h (onset of reaction) and 5 h. The abatement was determined by the change in the absorbance of CA at 273 nm, IBP at 221 nm, Ph at 270 nm, and DA at 237 nm. The photocatalytic activity of photocatalysts decreases according to the recalcitrance of organics (activity versus recalcitrance). With exception of cinnamic acid, rutile was more active than anatase and brookite at the onset of reaction and after 5 h it exhibited the similar high activity as those of anatase and brookite, and even with the commercial photocatalyst titania P25 (Degussa). In the degradation of IBP and Ph, brookite had slightly higher onset activity than anatase. This shows the potential of brookite in the degradation of hazardous organics regarding the water and wastewater treatment. Additionally, it confirms that the much less attention to brookite phase is not due to the activity but rather the metastable state. Also with the highest recalcitrant organic, DA was abated in the treatment with brookite.
Figure 3. Abatement of cinnamic acid (CA), ibuprofen (IBP), phenol (Ph), and diatrizoic acid (DA) obtained (a) 1 h and (b) 2 h in the photocatalytic performance of anatase (A), brookite (B), rutile (R), titania P25 (P25). Reaction conditions: RT, 10 ppm pollutant, 250 ml reaction solution, catalyst loading: 10 mg.
Download figure:
Standard image High-resolution imageA detail view on the photocatalytic performance of titania polymorphs was further studied by TOC (total organic carbon) measurement which determined the change in the amount of carbon presented in an organic compound after the photocatalytic treatment (table 2). Compared to high abatement (destruction of aromatic ring), the mineralization proceeds slowly (abatement versus mineralization). This indicates the formation of reaction intermediates. After 5 h, the mineralization was not complete. Only the comparatively high abatement and mineralization of CA was achieved in the treatment of titania P25, anatase, and brookite of 97–99% (UV–Vis) and of 92–97% (TOC). Such results show that the similar high activity of anatase and brookite is due to the similar structural and textural properties. They have small particle size and high specific surface area compared with the large crystal rutile. However, the high activity of rutile in the photocatalytic degradation of CA would be explained by the reactivity of this molecule, the nature of rutile surface. This also indicates particle size and surface area are not the limiting factors.
Table 2. The obtained mineralization of different pollutants over anatase (A), brookite (B), rutile (R), and titania P25 (P25) after the photocatalytic treatment for 1 h and 5 h.
| Pollutants | Mineralization (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 5 h | |||||||
| A | B | R | P25 | A | B | R | P25 | |
| CA | 17 | 30 | 23 | 35 | 92 | 93 | 69 | 97 |
| IBP | 25 | 29 | 7 | 40 | 84 | 85 | 28 | 91 |
| Ph | 11 | 17 | 3 | 33 | 47 | 42 | 8 | 95 |
| DA | 11 | 12 | 0 | 22 | 53 | 50 | 0 | 59 |
In the degradation of dyes rose bengal (RB) and rhodamine B (RhB), the photocatalytic decolourization and mineralization were also investigated in parallel. The decolourization was determined by the change in the intensity of colour band of RB at 524 nm and RhB at 554 nm. Figure 4 shows that the dye adsorption is much higher than the case of pharmaceuticals (7–38%). Especially, brookite had highest dye adsorption capacity for the more hydrophobic RB molecules even it has smaller BET surface area than anatase (table 1). Under UV irradiation, all titania samples exhibited the high decolourization of both dyes RB and RhB after 5 h. In contrast, mineralization is much less. In the previous reports, the decolourization of dyes occurs due to the dye self-photosensitization under the strong UV emitter Xe or Hg lamps combined to high dye loading [43, 44] which would inhibit the formation of active species formed by photogenerated electrons and holes on catalyst surface. With our testing conditions, a large amount of dyes was decolourized in the treatment with low catalyst concentration (catalyst to dyes ratio of 4) and low power of UV solarium irradiation. This was considered by the high contribution of the photocatalytic performance of synthesized photocatalysts. Because of the formation of reaction intermediates (hydroxylation products, partial oxidation products or ring-opened products), a part of dye molecules was degraded only leading to the relatively low mineralization. No mineralization was obtained in the treatment with rutile. This was reflected by the slightly lower abatement of aromatic ring compared to high decolourization. The abatement curve was determined by the change in the absorbance of RhB at 225 nm and RB at 260 nm, were not shown here. Therefore, the degradation of dyes was overestimated by considering the decolourization alone.
Figure 4. Decolourization and mineralization of (a) rhodamine B and (b) rose bengal over anatase (A), brookite (B), rutile (R), and titania P25 (P25). Reaction conditions: RT, 10 ppm dyes, 250 ml reaction solution, catalyst loading: 10 mg.
Download figure:
Standard image High-resolution image3.2.2. Intermediates.
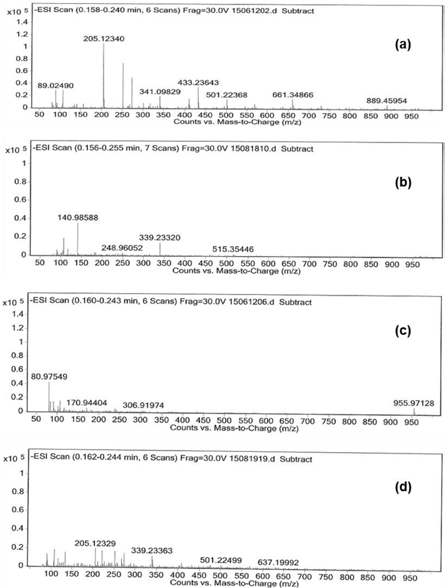
The incomplete mineralization of organics and the lower mineralization compared to high abatement of organics could be explained by the formation of reaction intermediates/by-products by means of ESI-TOF-MS (electrospray ionization time-of-flight mass) spectra in negative ionization mode. Figure 5 shows the negative ESI- TOF-MS spectra of starting IBP and IBP aqueous solution treated with three titania polymorphs under UV–vis irradiation for 5 h. The (M–H)− peak presented at m/z 205 is ascribed to the deprotonated IBP [13, 45, 46] (figure 5(a)). Beside this mass peak, lower and higher (M–H)− peaks appeared at m/z 89, 137, 433, 661, and 889, respectively, in the starting solution, are assigned to agglomerated IBP [13].
Figure 5. Negative ESI-TOF-MS spectra of (a) starting IBP solution and IBP solution treated with (b) anatase, (c) brookite, and (d) rutile after 5 h. Reaction conditions: RT, IBP 10 ppm, 250 ml reaction solution, catalyst loading: 10 mg.
Download figure:
Standard image High-resolution imageInterestingly, the (M–H)− peak at m/z 205 disappeared completely after 5 h in the treatment with both anatase and brookite. In contrast, it still presented with lower intensity in the treatment with rutile causing the lower conversion of IBP. The presence of mass peaks at 141, 249, 339, 515 (figure 5(b)), 81, 171, 307 (figure 5(c)), 339, 501, 637 (figure 5(d)) indicates the formation of oxidation IBP species. The presence (formation) of reaction intermediates explained the lower mineralization compared to the high abatement of aromatic ring. Also, it further confirmed the similar high activity of brookite compared to anatase, which is determined by UV–Vis and TOC.
3.2.3. Active species.
The study on the contribution of active species derived from the hole/radical trapping experiments could give a detailed view of the difference in the activity between three titania polymorphs. For this purpose, ethylenediaminetetraacetic acid (EDTA) acts as a hole scavenger [47, 48], tert-butanol (t-BuOH) acts as a •OH radical scavenger [49], and 1,4-benzoquinone (1,4-BQ) acts as a  radical scavenger [50]. Figure 6 and table 3 show the impact of active species (addition of scavenger) on the photocatalytic abatement of IBP over synthesized titania polymorphs at the onset of reaction (2 h). Without addition of scavenger, the activity decreased in the following order (abatements of IBP obtained after 2 h): anatase (87%) > brookite (83%) > rutile (31%).
radical scavenger [50]. Figure 6 and table 3 show the impact of active species (addition of scavenger) on the photocatalytic abatement of IBP over synthesized titania polymorphs at the onset of reaction (2 h). Without addition of scavenger, the activity decreased in the following order (abatements of IBP obtained after 2 h): anatase (87%) > brookite (83%) > rutile (31%).
Table 3. The photocatalytic abatement of IBP (%) over anatase (A), brookite (B), and rutile (R) after the addition of EDTA (hole scavenger), t-BuOH (OH scavenger), 1,4-BQ ( scavenger).
scavenger).
| Irradiation time (h) | EDTA | t-BuOH | 1,4-BQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | R | A | B | R | A | B | R | |
| 1 | 11 | 12 | 26 | 49 | 55 | 7 | 12 | 6 | 0.7 |
| 2 | 29 | 48 | 50 | 73 | 75 | 11 | 29 | 19 | 2 |
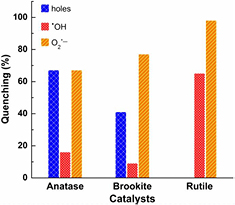
Figure 6. Quenching of the photocatalytic abatement of IBP over anatase, brookite, and rutile by addition of hole and radical scavengers under UV–Vis irradiation for 2 h. Reaction conditions: RT, IBP 10 ppm, 250 ml reaction solution, catalyst loading: 10 mg.
Download figure:
Standard image High-resolution imageThe highest impact of valence band holes (h+) exhibited in the treatment with anatase and followed by brookite which has similar activity. In contrast, the marginal impact of holes was observed with the lower active rutile. The formation of •OH radicals is highest with rutile, whereas it is minor with brookite. Surprisingly, superoxide ( ) radicals have a high impact with all titania polymorphs. According to previous studies, hydroxyl radicals (redox potential +2.8 V versus NHE, normal hydrogen electrode) are mainly important in the photocatalytic degradation of recalcitrant organics [15, 51–54]. However, the present work found the importance of holes for organic oxidation, especially at the onset of reaction. Due to the cleavage of aromatic ring by the attack of holes is required toward the complete mineralization of aromatics into CO2 as final oxidation product. This was reflected by the remarkably higher onset activity of anatase and brookite compared to rutile, whereby the degradation and mineralization are higher. The lack of holes might be reasonable causing the apparently lower photocatalytic activity of rutile TiO2.
) radicals have a high impact with all titania polymorphs. According to previous studies, hydroxyl radicals (redox potential +2.8 V versus NHE, normal hydrogen electrode) are mainly important in the photocatalytic degradation of recalcitrant organics [15, 51–54]. However, the present work found the importance of holes for organic oxidation, especially at the onset of reaction. Due to the cleavage of aromatic ring by the attack of holes is required toward the complete mineralization of aromatics into CO2 as final oxidation product. This was reflected by the remarkably higher onset activity of anatase and brookite compared to rutile, whereby the degradation and mineralization are higher. The lack of holes might be reasonable causing the apparently lower photocatalytic activity of rutile TiO2.
4. Conclusion
In summary, the photocatalytic performances of hydrothermally synthesized titania polymorphs were investigated under the reliable testing conditions (low power of solarium lamp equivalent UV, low catalyst concentration, and high organic loading). Such conditions are required for the degradation of aromatic pharmaceuticals and dyes presented in aqueous solution with low concentration. All titania polymorphs are active. The general activity order was obtained as follows: anatase ⩾ brookite > rutile. Depending on the reactivity or the recalcitrance of organics, they exhibited different activities. Usually, rutile has lower activity than anatase and brookite, which is considered due to the lack of oxidative holes determined by scavenger experiments. The crystal size and specific surface area are not limiting factors to explain the photocatalytic activity of rutile in the degradation of cinnamic acid. Rutile had slightly higher activity than those of anatase and brookite, close to that of titania P25 showing the nature of holes and active sites on rutile surface. Under the present synthesis condition, brookite which has similar structural and textural properties as anatase, is superior photocatalyst even with highly recalcitrant diatrizoic acid. Brookite and anatase have a lot of holes which favour the direct organic oxidation (the cleavage of aromatic ring) regarding the complete mineralization of organics into carbon dioxide (CO2) as final oxidation product. A complement use of UV–Vis spectroscopy and TOC analysis is required to achieve a comprehensive realistic assessment on the photocatalytic degradation of organic compounds.
Acknowledgments
This work has been supported by the European Union in the frame of the FP7 program (EU-project PCATDES). We deeply thank Dr Christine Fischer (LIKAT) for ESI-TOF-MS analysis, Dr Ursula Bentrup and MSc Thanh Huyen Vuong (LIKAT) for diffuse reflectance UV–vis measurements. We also thank Mrs Petra Duncker (University of Rostock) for technical assistance in TOC measurements and Dr Ung Thi Dieu Thuy (Institute of Materials Science (IMS), Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam) for supplying the SEM images of TiO2 samples.
Footnotes
- *
Invited talk at 8th International Workshop on Advanced Materials Science and Nanotechnology (IWAMSN2016), 8–12 November 2016, Ha Long City, Vietnam.