Abstract
Porous ceramic candle filters (PCCF) were prepared by sintering silica from rice husk with silver nanoparticles (AgNPs)/zeolite A at about 1050 °C to create bactericidal PCCF/AgNPs for water disinfection. The silver content in PCCF/AgNPs was of 300–350 mg kg−1 determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) and the average pore size of PCCF/AgNPs was of 50–70 Å measured by Brunauer–Emmett–Teller (BET) method. The bactericidal activity and silver release of PCCF/AgNPs have been investigated by flow test with water flow rate of 5 L h−1 and initial inoculation of E. coli in inlet water of 106 CFU/100 mL. The volume of filtrated water was collected up to 500 L. Results showed that the contamination of E. coli in filtrated water was <1 CFU/100 mL and the content of silver released from PCCF/AgNPs into filtrated water was <1 μg L−1, it is low, far under the WHO guideline of 100 μg L−1 at maximum for drinking water. Based on the content of silver in PCCF/AgNPs and in filtrated water, it was estimated that one PCCF/AgNPs could be used to filtrate of ∼100 m3 water. Thus, as-prepared PCCF/AgNPs releases low content of silver into water and shows effectively bactericidal activity that is promising to apply as point-of-use water treatment technology for drinking water disinfection.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Waterborne diseases caused by microorganisms lead to millions of deaths per year due to diarrhea. According to [1], over one billion people worldwide lack access to improved drinking water sources, and many more lack access to safe water as defined by the WHO risk-based guidelines for drinking water quality [2]. One of the reasons is that they do not have access to clean portable water sources [3]. Therefore, development of innovative water treatment technology is of the utmost importance. Porous ceramic water filters (PCWFs) have been considered as one of the most promising point-of-use (POU) water treatment technologies, especially for treatment of drinking water in the developing countries, and furthermore, PCWFs are low-cost and robust devices [4, 5]. However, one of the essential points is that PCWFs can only block bacteria and do not have adequately bactericidal property [6–8]. Therefore, research works of modification of PCWF to create bactericidal activity with silver nanoparticles (AgNPs) either only by impregnation of bare PCWF in colloidal AgNPs solution [9, 10] or by impregnation of PCCF treated with aminosilane coupling agent for linking AgNPs with PCWF [7, 8] have been carried out. Recently, Ren and Smith (2013) studied to compare the effectiveness of three treatment methods of PCWF with AgNPs, namely paint-on, dipping and fired-in (sintered-in) methods [11]. The obtained results indicated that the paint-on and dipping methods released about 1000 times more silver into filtrated water than the fired-in method. They concluded that the fired-in method might be a new and significant improvement to produce antimicrobial PCWF. Results of our previous study on treatment of porous ceramic candle filter (PCCF) with aminopropyltrietho-xysilane (NH2-C3H6-Si(OC2H5)3) for fixing AgNPs onto PCCF through coordination bonds between –NH2 groups of the amino-silane and the silver atoms of AgNPs showed that silver release into filtrated water was less than 10 μg L−1, and it is far below the WHO guideline of 100 μg L−1 at maximum for drinking water [8]. In addition, the contamination of Escherichia coli (E. coli) in filtrated water was of <1 CFU/100 mL, which meets the WHO risk-based guidelines for drinking water quality of fecal and coliform counts in drinking water [2]. Thus, the PCCF impregnated with AgNPs with the silver content of 200–250 mg kg−1 exhibited highly antimicrobial activity, so it can be applied for POU drinking water treatment [8]. However, the process of treatment of PCCF with AgNPs through coupling agent was rather labor intensive and seemed not to be competitive in terms of production cost, which restricted its application for large-scale production.
In this study PCCFs were produced by sintering silica from rice husk with AgNPs deposited on zeolite 4 A and together with binding and foaming agents at 1050 °C (PCCF/AgNPs). The release of silver from PCCF/AgNPs into filtrated water and bactericidal activity of PCCF/AgNPs against E. coli was carried out by flow test. The effective filtration time of PCCF/AgNPs was also estimated.
2. Experimental
2.1. Production of bactericidal PCCF/AgNPs
AgNPs deposited on zeolite 4 A (AgNPs/Z) is a product prepared by our research group according to the method of [12], but using hydrazine hydrate (N2H2 H2O) instead of sodium borohydride (NaBH4) as reducing agent.
AgNPs size was determined by x-ray diffraction (XRD) on D8 Advance Brucker, Germany. UV-Vis absorption spectrum of AgNPs/Z dispersed in 2% polyvinyl alcohol (PVA) solution was taken on the Shimadzu UV-1650 PC spectrophotometer, Japan. AgNPs content in AgNPs/Z and in PCCF/AgNPs was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) on a Perkin-Elmer, Optima 5300 DV. The presence of silver in PCCF/AgNPs was also assessed by energy dispersive x-ray spectroscopy (EDX) on a JEOL 6610 LA.
PCCF/AgNPs samples were produced by sintering silica from rice husk with AgNPs/Z at 1050 °C at a domestic Ceramic Co., Hai Duong province, Vietnam. The average pore size was measured by Brunauer–Emmett–Teller (BET) method (Quantachrom Nova 1200) using N2 as the adsorbate.
2.2. Flow test of silver release from PCCF/AgNPs
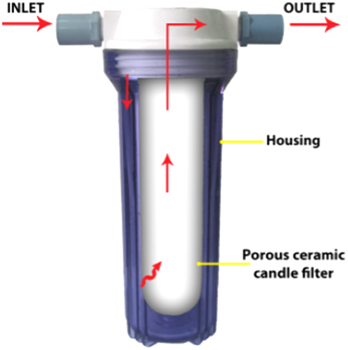
The PCCF/AgNPs was connected to tap water with the flow rate of ∼5 liters h−1 up to 500 liters. The filtrated water samples were collected for determination of the silver content by neutron activation analysis method at Dalat nuclear research reactor and by inductively coupled plasma-mass spectroscopy (ICP-MS) on an Optima 7300 DV instrument (Perkin-Elmer, USA). The model of PCCF/AgNPs mounted in its housing for flow test is briefly described in figure 1.
Figure 1. Model of PCCF mounted in its housing for flow test.
Download figure:
Standard image High-resolution image2.3. Bactericidal activity
2.3.1. In vitro test
The Luria–Bertani (LB) medium for bacteria incubation was purchased from Himedia, India. The Escherichia coli ATCC 6538 (E. coli) was provided by University of Medicine-Pharmacy in Ho Chi Minh City. To examine the bactericidal activity of PCCF/AgNPs, 1 mL of ∼107 CFU mL−1 (CFU: colony-forming units) E. coli suspension was separately added to 99 mL LB medium in 3 conical flasks (250 mL). The cultures were shaken at 150 rpm for 20 min at room temperature. Then 1.7 g of PCCF/AgNPs powder was introduced into one tested flask with silver content of about 5 mg L−1. The same weight of bare PCCF powder was added to the second flask, and the third flask was used as the blank control. All flasks were shaken at 150 rpm for 30 min, and then E. coli suspensions were diluted tenfold in distilled water to 10−5 of initial concentration. 0.1 mL of each diluted solution was spread on LB agar plates, and incubated at 37 °C overnight (∼16 h). The counts of bacterial colonies were the surviving numbers of E. coli [13].
2.3.2. Flow test
The inlet water as described in figure 1 was inoculated with E. coli of ∼106 CFU/100 mL. The bactericidal activity of PCCF/AgNPs was also investigated with the flow rate of ∼5 L h−1. The output water passed through PCCF/AgNPs was collected up to 500 L for assessment of the E. coli contamination according to ISO 9308-1: 2000 [14].
3. Results and discussion
3.1. Characteristics of AgNPs/zeolite
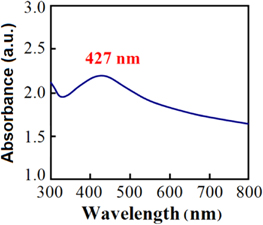
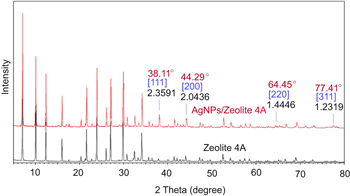
Figure 2 shows the maximum absorption wavelength (λmax) of AgNPs/zeolite at 427 nm. Shameli et al also reported the characteristics of their AgNPs/zeolite products with the λmax values in the range of around 394–401 nm that correspond to AgNPs smaller than 10 nm [12]. From the XRD pattern in figure 3, the average size of the metallic AgNPs deposited on zeolite was calculated using the peak at 2θ = 38.11° with full width at half maximum (FWHM) of about 0.277 and based on Debye–Scherrer's formula: t = 0.9λ/Bcos θ as described by Jiang et al [15].
Figure 2. UV-Vis spectrum of 0.5% AgNPs/zeolite in 2% PVA solution.
Download figure:
Standard image High-resolution imageFigure 3. XRD patterns of zeolite 4 A and AgNPs/zeolite 4 A.
Download figure:
Standard image High-resolution imageAs the result of calculation, the size of AgNPs was of about 30 nm. The content of AgNPs deposited on zeolite 4 A analyzed by ICP-AES was of about 1.2% (w w−1). Results of our previous study on doping AgNPs onto silica by gamma 60Co irradiation method indicated that the size of AgNPs determined also by XRD was of about 23 nm, which was in fair correlation with particles size estimated from transmission electron microscopy (TEM) images [16]. Both doped products namely AgNPs/Z and AgNPs/SiO2 have almost the same AgNPs content of around 1.0–1.2% (w w−1).
3.2. Characteristics of PCCF/AgNPs
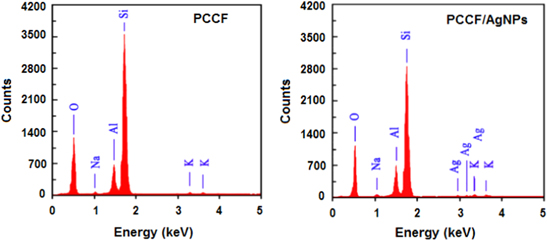
Results of energy dispersive x-ray (EDX) spectra in figure 4 showed that the composition of PCCF consists of three main elements, particularly silicon, aluminium, oxygen and a small amount of sodium and potassium, but without any trace of silver. After sintering with AgNPs, the peak at 3 keV appeared in EDX spectrum confirming the presence of silver in the composition of PCCF/AgNPs. In the study of Klemenčič et al [17] and Gusseme et al [18], the EDX spectrum was also used to confirm the presence of AgNPs in cellulose and polyvinylidene fluoride samples. Table 1 described some characteristics of as-prepared PCCF/AgNPs.
Figure 4. EDX spectra of PCCF and PCCF/AgNPs.
Download figure:
Standard image High-resolution imageTable 1. Some characteristics of PCCF/AgNPs.
| Parameters | Values |
|---|---|
| Length (mm) | 200–202 |
| Inner diameter (mm) | 30.4–30.6 |
| Outer diameter (mm) | 50.6–50.8 |
| PCCF/AgNPs weight (g) | 300–350 |
| Mean pore size (μm) | 0.005–0.007 |
| BET (m2 g−1) | 1.5–2.5 |
| AgNPs content (mg kg−1) | 300–350 |
For the condition of PCCF/AgNPs production, the sintering temperature was at 1050 °C, exceeding the melting point of silver 961 °C [11]. It is expected that at this temperature, AgNPs will be strongly cemented to the walls of porous ceramic and sintering in air medium; some certain content of AgNPs reacts with oxygen to form Ag2O nano and/or Ag2O/AgNPs clusters.
The XRD patterns of PCCF/AgNPs and PCCF in figure 5 indicated that the AgNPs still remained and were partly oxidized to form Ag2O nano during the sintering process. Sullivan et al [19] also reported XRD peaks of AgNPs and Ag2O nano in their study of the synthesis of nanocomposite material. Further study of the effect of sintering temperature on the formation of Ag2O nano should be carried out. Lalueza et al [20] reported the bacterial effects of different silver-containing materials that are in the following sequence: AgNO3 > silver-exchanged zeolite > Ag2O > commercial silver-exchanged zeolite (granular) >commercial silver-exchanged zeolite (pellets) >AgNPs 100 nm. Liu and Hurt [21] claimed that AgNPs can usually be oxidized in aqueous solution when exposed to air (equation (1)), which results in the release of silver ion under acidic conditions (equation (2)) as follows:
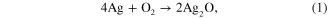
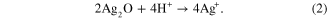
Figure 5. XRD patterns of PCCF and PCCF/AgNPs.
Download figure:
Standard image High-resolution imageAccording to Sotiriou and Pratsinis [22], the mechanism of AgNPs toxicity to bacteria depends strongly on Ag+ release, and Ag+ is the definitive molecular toxicant. In recent years, several authors reviewed possible mechanisms of AgNPs [23, 24], but the exact mechanism is still not fully elucidated. It was generally believed that silver ions interact with bacterial cell wall, plasma membranes, bacterial DNA and protein, as well as ribosomes, resulting in bactericidal effects.
Therefore, silver ions released from AgNPs have been proven to be one possible mechanism; however, it is not the only mechanism for the antimicrobial activity of AgNPs. Thus, it is expected that Ag+ is formed from AgNPs, Ag2O and/or Ag2O/AgNPs clusters when PCCF/AgNPs is in contact with water, and therefore Ag+ will be the main bactericidal agent during water filtration.
3.3. Content of silver release from PCCF/AgNPs into filtrated water
Results in table 2 proved that the contents of the silver released from PCCF/AgNPs in the filtrated water by flow test with the rate of ∼5 L h−1 were less than 1 μg L−1 determined by neutron activation analysis (NAA) and ICP-MS methods. The contents are far below the WHO guideline of 100 μg L−1 silver at maximum for drinking water [2].
Table 2. The content of silver released from PCCF/AgNPs in filtrated water by flow test.
| Volume of filtrated water (L) | 0 | 25 | 50 | 100 | 200 | 300 | 400 | 500 | |
|---|---|---|---|---|---|---|---|---|---|
| Ag content (μg L−1) | ICP-MS | 0.012 |
0.013 | 0.016 | 0.015 | 0.045 | 0.019 | 0.016 | 0.018 |
| NAA | — | 0.011 | 0.027 | 0.020 | 0.028 | 0.067 | 0.053 | 0.082 | |
Results in table 2 also indicate that both methods for determination of silver in water are in accordance with each other. Oyanedel–Craver et al [9] and van Halem et al [10] also studied silver release from silver-impregnated porous ceramic pot filter for low cost household drinking water treatment. However, they did not used a coupling agent like aminosilane to fix AgNPs to the ceramic wall, therefore silver was easily leaching from the pot and the bactericidal effect should be quickly decreased with the filtration time. Thus, PCCF/AgNPs showed less silver release (∼1 μg L−1) compared to silver-impregnated porous ceramic even treated with coupling agent aminosilane (∼10 μg L−1) [8].
3.4. Antimicrobial activity
3.4.1. In vitro test
The surviving number of E. coli in the tested medium was of 15 × 105; 5.8 × 105 and 0 CFU ml−1 for control, PCCF and PCCF/AgNPs samples, respectively, as shown in figure 6. The in vitro test results indicated that PCCF/AgNPs has highly bactericidal activity against E. coli.
Figure 6. E. coli colonies forming on LB agar: (A) control, (B) PCCF, and (C) PCCF/AgNPs.
Download figure:
Standard image High-resolution image3.4.2. Flow test
The results in table 3 indicated that the water filtrated though PCCF/AgNPs up to 500 L was not contaminated by E. coli (<1 CFU/100 mL), which is generally accepted for drinking water [2] in comparison to 2.5 × 104 CFU/100 mL (up to 50 L) for blank PCCF (data not shown).
Table 3. The counts of E. coli in filtrated water by flowing test though PCCF/AgNPs.
| Volume of filtrated water (L) | 25 | 50 | 100 | 200 | 300 | 400 | 500 |
|---|---|---|---|---|---|---|---|
| E. coli (CFU/100 mL) | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
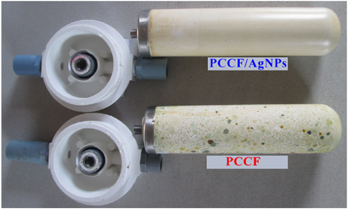
Figure 7 shows that the outside of the PCCF/AgNPs had almost no growth of microorganisms compared to the bare PCCF one. This observation confirmed again the highly antimicrobial activity of the PCCF/AgNPs.
Figure 7. The outside appearance of PCCF and PCCF/AgNPs after use in water flow test.
Download figure:
Standard image High-resolution imageAmong the water treatment materials, ceramic filters (disk, candle and pot) proved to be one of the best treatment options for reducing bacteria by more than 99% [5]. According to Sui and Huang [25] the PCCF can be used to filtrate more than 50 M3 of drinking water with the regularly mechanical brush to maintain the stable flux [25]. Our PCCF/AgNPs product contained of about 100 mg AgNPs. Based on the silver content released from the PCCF/AgNPs into water of about <1 μg L−1 as presented in table 2, it can be theoretically calculated that one PCCF/AgNPs could be used to filtrate about 100 m3 water.
Recently, Abebe et al [26] reported that household-level ceramic water filter intervention can improve drinking water quality and decrease days of diarrhea for people living with the human immunodeficiency virus in rural South Africa. Therefore, the as-produced PCCF/AgNPs with highly bactericidal activity can be recommended to the communities to apply for POU drinking water treatment.
4. Conclusion
The bactericidal PCCFs/AgNPs were produced by sintering silica from rice hash with AgNPs/zeolite A at about 1050 °C. Results of flow test with the rate of ∼5 L h−1 on silver release and antimicrobial effect for E. coli of PCCF/AgNPs indicated that the silver content in filtrated water was less than 1 μg L−1, which meets the WHO guideline of 100 μg L−1 for drinking water, and the contamination of E. coli was of <1 CFU/100 mL. In addition, the sintering method is less labor intensive than the impregnation method. Therefore, the PCCF/AgNPs products with silver content of 300–350 mg kg−1 that have allowable low content of silver released into filtrated water and highly bactericidal activity can be favorably applied for household treatment of drinking water.
Acknowledgements
We gratefully thank Mr Nguyen Trong Viet for his cooperation of PCCF/AgNPs production. This research is supported in part by Vietnam–Hungary research collaboration project, Code no. 20/2011/HD-NDT.