Abstract
Light-triggered treatments in the presence of photosensitizer and oxygen molecule are prevalent for a wide variety of diseases. The reactive oxygen species (ROS), which are produced in the vicinity of malignant tissue by the light activated sensitizers, are considered as active agents in the photodynamic therapy (PDT). The higher reactivity of photosensitizers in nano state enhances the profile of pharmacokinetics and therapeutic index of the active molecule. In that direction, this review emphasises the application of porphyrin (Por) and phthalocyanine (PC) as nano photosensitizers in the development of antibacterial nanotherapeutic agents for resistant and non-resistant bacteria.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The growing antibacterial resistance among pathogens [1] remains a global threat to health care as an endemic which keeps reminding scientific societies to develop new antibiotics. The interest towards the development of conventional antibiotics is reduced due to continual problems of bacterial resistance. Therefore, it is an emerging interest in the design and development of new materials with a novel and distinct mechanism in killing pathogens by replacing the conventional mechanisms. In a recent scenario, among the several alternative approaches, photodynamic therapy (PDT) is being used as one of the most promising clinical strategies to treat pathogenic, particularly bacterial, infections with a new type of therapy called photodynamic antimicrobial chemotherapy (PACT).
For a long time light activated compounds have been well known for light-triggered treatments of a wide variety of diseases, particularly cutaneous infections, which were exposed to sunlight with photoactivated compounds in anticipation of healing the infected zone, and the method for the treatment of infections was accepted as proof of principle [2–4]. In this context, the Nobel Prize was awarded to Niels Finsen in 1903 to control skin manifestation of tuberculosis using light treatment which convinced us of the magnitude of potentiality and applicability as one of the promising approaches for treating diseases [5]. Therefore, light as a promising external agent could trigger a special class of chemical compounds to convert tissue 3O2 into the reactive oxygen species (ROS) in the vicinity of the infected cells which ultimately leads to cell death. These special classes of chemical compounds, which become activated on exposure to light are known as photosensitizers. Thus, the technique of multimodality treatment combining the preferential accumulation of photosensitizer (PS) in the malignant tissue or cells with the precise wavelength of light and the inherent tissue oxygen (3O2) is known as photodynamic therapy (PDT); the light penetrates the cells of tissue and causes excitement of the photosensitizer. In this treatment, activation of the photosensitizers by light and light transmission through tissue are pre-requisites to successful PDT. A specific wavelength of light is required for each photosensitizer to maximize penetration through the tumour and excitation of the photosensitizer. Thus, the role of photosensitizer is inevitable in PDT in which the sensitizing agent becomes activated successfully by light energy in the presence of oxygen.
The families of photosensitizers can be classified by their chemical structure: porphyrins (hematoporphyrin, benzoporphyrin, texaphyrins), chlorophylls (chlorins, purpurins, bacteriochlorins), and dyes (phthalocyanine, napthalocyanine). Photosensitizers possess a stable singlet electronic configuration in their lowest or ground state energy level. In the presence of a particular wavelength of light, a photosensitizer is stimulated to an excited singlet state for a short period and it returns to the ground state in the absence of light either by emitting fluorescence or by internal conversion with loss of energy in terms of heat. In addition to the above concept, the molecule, i.e. photosensitizer, may convert to the triplet state by a change in the spin of an electron via intersystem crossing. The triplet state photosensitizer has lower energy than the singlet state, but has a longer period of lifetime (>500 ns). As a result, this particular feature of the photosensitizer increases the possibility of energy transfer to the adjacent oxygen molecules. The tendency for a conversion of photosensitizer into the triplet state is measured by the triplet state quantum yield, which measures the probability of formation of the triplet state per photon absorbed. The lifetime of triplet state influences the amount of cytotoxic species produced by collision-induced energy transfer to molecular oxygen and other cellular components such as amino acids (particularly cysteine, histidine, tryptophan, tyrosine and methionine), nucleosides (mainly guanine) and unsaturated lipids which can react with singlet oxygen. A high probability of intersystem crossing will produce an effective population of excited triplet state photosensitizer molecules whose energy can then be transferred by the two mechanisms which are difficult to distinguish. Type I which involves electron transfer and/or type II which follows energy transfer reactions are proposed to generate highly reactive oxygen species (ROS), resulting in necrosis and/or apoptosis of exposed cells [6–8]. Typically, type I reactions generate radical and radical anion species (e.g., O2•−, HO•), while type II reactions produce reactive singlet oxygen (1O2). For the type II pathway, the impact of PDT is highly dependent on the oxygen content. In cancer therapy, the inner region of a tumor is commonly hypoxic [9] due to insufficient blood supply. In addition, oxygen shortages can occur as a result of photochemical consumption and vascular damage during PDT, which further limits the efficacy in tumor destructions [10]. The type I mechanism involves hydrogen-atom abstraction or electron-transfer between the excited PS and a substrate, yielding free radicals [11]. These two competing mechanisms can occur simultaneously [12]. It is generally believed that 1O2 formed from a type II reaction is primarily responsible for the biological PDT effect [13, 14]. However, several recent studies indicate that radical species from the type I mechanism may lead to an amplified PDT response, particularly under low oxygen conditions [15, 16]. Direct comparisons between the contributions of type I and type II mechanisms to PDT efficacy are difficult due to the complexity of ROS formation on environmental factors. However, despite of the above-mentioned phenomena, photodynamic therapy (PDT) is an emerging clinical modality that has received considerable attention for the treatment of bacterial, cancer, cardio-vascular, dermatological, and ophthalmic diseases [17–19]. New photosensitizing drugs (porfimer sodium, photochlor) based on porphyrins and chemically related compounds such as chlorins and phthalocyanines have been under extensive investigation [20, 21]. Most photosensitizers are hydrophobic and poorly soluble in water; to the best of our knowledge, this problem related to the solubility could be encountered by converting the bulk materials into nanomaterial. In that direction, a variety of nanostructured materials such as polymer conjugates [22], polymeric micelles [23, 24], liposomes [25] and ceramic nanoparticles [26] have been successfully studied to improve drug availability for parenteral administration, and to further increase nanoparticle uptake for enhanced therapeutic efficacy. Despite these exciting advances, few reported studies have been focused on the carrier–drug interactions, and in particular, how the carrier microenvironment may affect the photophysical properties of PS-based drugs, and subsequently, their biological efficacy in killing malignant cells. Therefore, it is an emerging interest in the design and development of new materials with a novel and distinct mechanism in killing bacteria by replacing the conventional mechanisms. In a recent scenario, among the several alternative approaches, PDT could be used as one of the most promising clinical strategies to treat bacterial infections with a new type of therapy called photodynamic antimicrobial chemotherapy (PACT). This unique approach has also been accepted in the treatment of a number of medical issues including skin, age-related macular degeneration (AMD), cancers, and viral infections [18]. In principle, the light source triggers or activates a photosensitizer depending on the wavelength of the light involved [27, 28]. On activation with light, the photosensitizer converts the oxygen of the concerned tissue into reactive oxygen species (ROS) such as singlet oxygen (1O2), hydroxyl radical and superoxide anion [29]. Out of different reactive oxygen species (ROS), it is obvious that singlet oxygen (1O2) is produced as a main species which is responsible for cell death in PACT. Along with these phenomena, it seems that the initially generated singlet oxygen interacts with some component of the cell to produce ROS [30]. Indeed the sensitivity of microorganisms to PACT has been tested against a range of Gram-positive and Gram-negative bacteria, fungi, enveloped and non-enveloped viruses [31]. Although, numerous light-activated antimicrobial agents have been reported, including titania and its alloys [32], methylene blue [33], toluidine blue [34].
Henceforth, to overcome the antibacterial resistant, the exciting area of research is looking at the use of PDT along with current therapy for bacterial infections to make it more effective. One way to do this may be to use PDT during surgery to help keep cancer from coming back on large surface areas inside the body, such as the pleura (lining of the lung) and the peritoneum (lining of the belly or abdomen). These are common sites of spread for some types of cancer. The adequate literature regarding the application of PDT in the treatment of cancer has been well documented in review articles. But it is fortunate for us that the review article on the use of PDT for the treatment of bacterial and fungal infection is not yet focused by the researchers. In addition, beneficiaries of the PS molecules in nano state due to ultra-small size, large surface-to-mass ratio, high reactivity and unique interactions with biological systems are established. The photosensitizer in nano state could be carried through physical encapsulation, adsorption, or chemical conjugation which assists change in the pharmacokinetics and therapeutic index. Thus, this review will explore the role of photosensitizers in the development of light-triggered nanoparticles for the treatment of different kinds of diseases, particularly the bacterial infections.
2. Porphyrin and phthalocynines as photo-sensitizers in photodynamic therapy
Porphyrins and phthalocyanines have been widely investigated as a vital part of photodynamic therapy (PDT) [35] producing reactive oxygen species (in particular, 1O2) upon exposure to light in the presence of oxygen [36]. Biocompatible porphyrins and their derivatives have extended conjugated electronic structure, largely visible to NIR absorption extinction molar constant, and acceptable fluorescent quantum yields. These features of porphyrin and phthalocyanine-derived nanoconjugates means they find wide application in biomedical fields including PDT, triplet sensitizer [37], fluorescent imaging probe [38], as well as oxygen carriers [39]. These aromatic organic compounds are a group of naturally occurring intensely coloured heterocyclic compounds, whose name is coined from the Greek word porphura (purple) [40]. These molecules are involved in a number of biologically important roles, including oxygen transport and photosynthesis. In addition, they also find wide applications in a number of fields, ranging from fluorescence imaging to medicine. The fundamental porphine framework consists of four pyrrolic sub-units linked on opposing sides through four methine (CH) bridges known as the meso-carbon atoms. If the meso-and/or β-hydrogens are substituted with non-hydrogen atoms or groups, the resulting compounds are known as porphyrins. These molecules show very good and sensitive electrochemical, photochemical, catalytic and biochemical activities. It is found that the intensity and colour of porphyrins are derived from the highly conjugated Π-electron systems and the most fascinating feature of porphyrins are their characteristic UV-visible spectra that consist of two distinct regions: in the near ultraviolet and in the visible region (figure 1). Natural and synthetic porphyrins have relatively low toxicity in vitro and in vivo. The ability for numerous chemical modifications and the large number of different mechanisms by which porphyrins affect microbial and viral pathogens, have placed them into a group of compounds with outstanding potential for discovery of novel agents, mechanisms and materials. Porphyrins have been extensively studied over the past decade for their potent biocidal capacity. Usually, porphyrins are more toxic toward Gram-positive bacteria, such as Staphylococcus aureus, which have weaker cell walls compared to Gram-negative bacteria such as Escherichia coli [41].
Figure 1. Porphyrin (Por) and phthalocyanine (PC).
Download figure:
Standard image High-resolution imageAnother class of photosensitizers, closely related to porphyrins (Pors) is phthalocyanines (PCs, figure 1), first characterized in the 1930s by Linstead and Robertson [42], which are intensely coloured, symmetric and aromatic macrocycles. The term 'phthalocyanine' was conceived from the structural origin of the phthalic acid precursor and 'cyanine' due to its colour. A number of substitution positions are available on phthalocyanine, either at the non-peripheral (α) or peripheral (β) positions of the MPc macrocyle, or through axial substitution on the central metal which facilitates these molecules' applicability to a wide variety of different scientific fields [43]. Due to coordination with a wide range of metals (via the central cavity) forming metal complexes, PCs show intensely coloured blue-green compounds, and widely used as colourants. PCs are planar, tetrapyrolic, macrocyclic aromatic compounds and possess an 18 π-electron, also having an extended conjugated pathway relative to porphyrins due to a fused benzene ring to the β-positions of each of the four pyrrolic sub-units. These benzene rings favour strengthening the absorption of the chromophore at longer wavelengths (670–780 nm) in visible light range with respect to class of porphyrins. PCs demonstrate stronger absorption of red light (than photofrin), allowing more effective light penetration to tumours cells; hence, these compounds constitute a second generation of photosensitizers [44]. Nowadays the derivatives of Por and PCs are also finding their utility as photoconducting materials in laser printers and the light absorbing layer in recordable compact discs. Other applications of these materials include as photosensitizers in phototherapy, fluorescent probe in vitro and in vivo, non-linear optical materials and industrial catalysts [45]. Phthalocyanines are an effective photosensitizer for the inactivation of various microbial pathogens [46]. It is found that Gram positive bacterial cells are more susceptible to the photodynamic inactivation (PDI), while Gram negative cells are significantly resistant to the photosensitizers normally used for PDT [47]. Thus, the importance of phthalocyanine molecules is reflected in industry, medicine and biology [48].
Although photodynamic therapy, as well as biologically active nanoparticles, are becoming potential alternatives for the treatment and eradication of emerging bacterial resistance to antibiotics, classical antiviral, antifungal, and antiprotozoal drugs [49] in clinical field, they could also be applicable in environmental management, more specifically for inactivation of pathogenic microorganisms in water and waste water [50].
3. Application of nanotechnology in medicine
The applications of nanotechnology [51] in medicine have been extensively explored in many medical areas, especially in drug delivery for the last few decades including gene and drug delivery [52, 53], as fluorescent biological labels or bio-imaging agents [54], magnetic resonance imaging (MRI) contrast enhancers [55], probes of DNA structures [56], bio-sensors [57–59], tumour destruction by heating (hyperthermia), and in tissue engineering [60]. Nanotechnology concerns the understanding and control of matters in the 1–100 nm range, at which scale materials have unique physicochemical properties including ultra-small size, large surface-to-mass ratio, high reactivity and unique interactions with biological systems. By loading drugs into nanoparticles through physical encapsulation, adsorption, or chemical conjugation, the pharmacokinetics and therapeutic index of the drugs can be significantly improved in contrast to the free drug counterparts. Many advantages of nanoparticle-based drug delivery have been recognized in science by improving serum solubility of the drugs, prolonging the systemic circulation lifetime, releasing drugs at a sustained and controlled manner; preferentially, delivering drugs to the tissues and cells of interest and concurrently delivering multiple therapeutic agents to the same cells for combination therapy. Moreover, drug-loaded nanoparticles can enter host cells through endocytosis and then release drug payloads to treat microbes-induced intracellular infections. Several biocidal materials such as nanoparticles [61] carbon nanotubes [62], and polymers containing pendant organic functionalities [63] have been tested in antibacterial therapies. The use of nanoparticles as a potential vehicle for photosensitizers has recently become a focus of interest in the field of photodynamic therapy [64]. As porphyrin and phthalocyanines are highly useful as antibacterial agents acting through ROS mechanisms, their application as nanoparticles should be exhibited to a higher extent than porphyrin or and phthalocyanine as bulk materials. The unique properties of nanoparticles allow obtaining photosensitizing nanodevices with improved photostability, favourable circulation half-life, and other physicochemical properties such as solubility, zeta potential surface/volume ratio etc. Thus, photosensitizers associated to nanoparticle could be explored in multifunctional platform as phototherapeutic/photodiagnostic agents. The conjugation of nanoparticles (quantum dots, iron oxide, silver, gold etc) to phthalocyanines/porphyrin is now receiving a great deal of attention [65] because the photoactivity under UV-irradiation [66] of these nanoparticles enhances the photochemical activity upon combination with PCs/Pors [67]. The high surface-to-volume ratio, quantum size effect, and electrodynamics interactions allows application of nanoparticles in various pharmaceutical arenas. Inorganic applications have already developed in various pharmaceutical fields but due to toxicity and less bioavailability, the possibility is offered to develop organic nanoparticles which are biocompatible, biodegradable, easily modified, with ease of drug delivery and also a controlled and/or targeted drug release. Hence, to develop novel therapeutics with less toxicity and more bioavailability and biocompatibility, either organic nanoparticle or the hybrid of organic and inorganic nanoparticles are in demand. This review will discuss the different research using porphyrin, an organic molecule for the preparation of light activated with organic or organic–inorganic hybrid nanoparticles for the development of novel therapeutics. The nanoparticles might be used for physical encapsulation, adsorption, or chemical conjugation. This mini-review focuses mainly on the synthesis, characterization and application of porphyrin and phthalocyanines including their derivatives as antibacterial nanoparticles.
4. Antibacterial nanoparticles photosensitizers by porphyrins and phthalocyanines
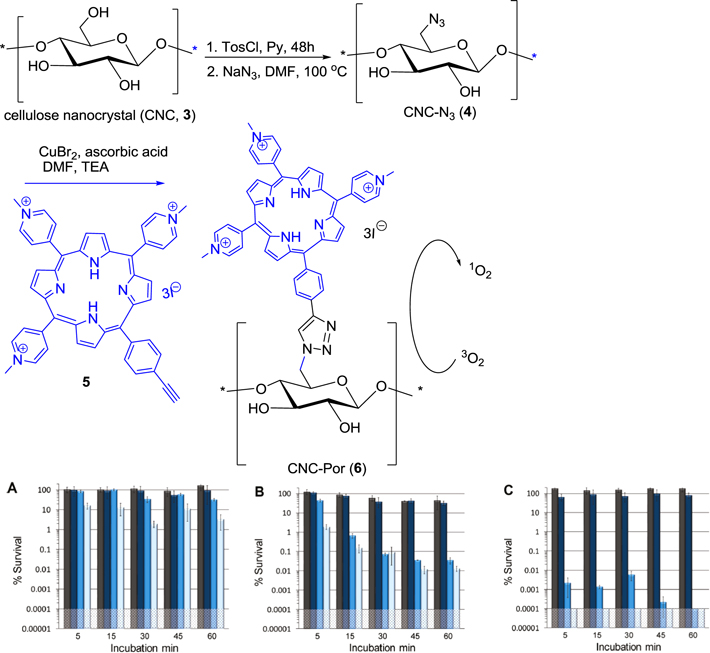
Ghiladi et al [68] were involved with an assignment to prepare a new type of photosensitized antibacterial nanoparticle using porphyrin as photosensitizer. In their research programme, the porphyrin-cellulose based nanocrystal has been selected to fabricate for photodynamic antimicrobial chemotherapy to antibiotic-resistant pathogens. From a synthetic point of view, the photoactive porphyrin was intercalated with cellulose nanoparticle surface through a triazole linkage which was formed via azide-alkyne Cu(I) catalyzed Huisgen 1,3-dipolar cycloaddition reaction between alkyne attached with porphyrin and azide on cellulosic surface.
The synthetic procedure involved preparation of rod-shaped cellulose nanocrystal (CNC) with an average length of 100–400 nm from Whatman #1 filter paper (98% α-cellulose, 80% crystallinity) via acidic hydrolysis. The next focus was the surface modification of cellulose nanocrystals (CNC, 3) (figure 2) with the cationic porphyrin (Por) (5) through a covalent bonding yielding the conjugated CNC-Por (6). The covalent attachment of the porphyrin to the surface of the cellulose nanocrystals potentially increased the longer lasting or permanent antimicrobial properties to the cellulose (improved durability), minimizing the percolation of the biocidal agent into the surrounding environment, and prevention of porphyrin aggregation. Modification of the primary hydroxyl groups of the CNC for the installation of surface azide groups was done in two steps: surface tosylation followed by azidation to obtain CNC-N3 (4). Attachment of the porphyrin onto the cellulose nanocrystalline surface was accomplished by click reaction with CuBr2/ascorbate in DMF at elevated temperature in the presence of triethylamine providing CNC-Por (6) in good yield. The resulting, generally insoluble, crystalline material, CNC-Por (6), was characterized by infrared and diffusion 1H NMR spectroscopies, gel permeation chromatography, and thermogravimetric analysis.
Figure 2. Synthesis and photodynamic inactivation of (A) E. coli (B) M. smegmatis and (C) S. aureus using 20 μM CNC-Por (6) as the photosensitizer.
Download figure:
Standard image High-resolution imageFor evaluation of their biological efficacy, photodynamic inactivation experiments of CNC-Por (6) as a photosensitizer on solid support were executed. Although in an aqueous system, CNC-Por (6) remains suspended, and not dissolved, it showed excellent efficacy toward the photodynamic inactivation of Mycobacterium smegmatis (M. smegmatis) and Staphylococcus aureus (S. aureus), but with only slight activity against Escherichia coli (E. coli).
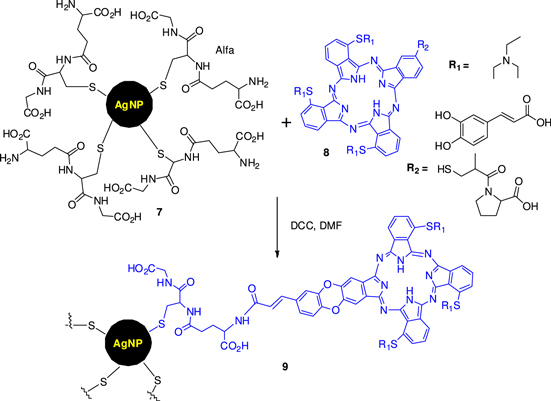
In recent times, phthalocyanines (PCs) have been recognised as efficient photosensitizers and have been successfully used in photodynamic therapy (PDT). Nomasonto et al [69] have taken initiative to prepare the hybrid of PCs with effective biocidal silver nanoparticles (AgNPs) anticipating synergic biological activity due to the combination of PACT (using the photosensitizing abilities of PCs) and AgNPs alone (with the antimicrobial properties). A low symmetry zinc phthalocyanine and glutathione-capped silver nanoparticles (GSH-AgNPs) (7) have been studied by this group. In their synthetic process, the AgNPs were prepared using an aqueous solution method from AgNO3 and stabilized by glutathione having two carboxyl moieties and an amino functional group for further conjugation of the AgNPs to phthalocyanines. As shown in figure 3, monocarboxy zinc phthalocyanines (ZnMCafPC, 8a, and ZnMCapPC, 8b) were coupled with the amino functions of GSH-AgNPs in the presence of DCC coupling reagents to get nanoconjugate 9. The conjugate was separated from any unconjugated nanoparticles by running the solution through a size exclusion column (Bio-Beads S-X1 from Bio-Rad) using THF as an eluent.
Figure 3. Schematic representation of the coupling reaction with glutathione-capped silver nanoparticle to low symmetry metallophthalocyanines.
Download figure:
Standard image High-resolution imageThe compounds were characterized by UV–vis, surface plasmon resonance (SPR) study, transmission electron microscopy (TEM) and Fourier transmission infrared (FTIR). The conjugation of metallophthalocyanines (MPCs) to AgNPs led to an improved photophysical and photochemical performance (triplet state, singlet oxygen, fluorescence quantum yields and lifetimes) of the photosensitizers, which enhanced photodynamic antimicrobial chemotherapy (PACT). The conjugates showed improved light toxicity, especially at 5–10 mM, displaying effectiveness as bactericides. These conjugates showed antimicrobial activity against E. coli in the presence and absence of light.
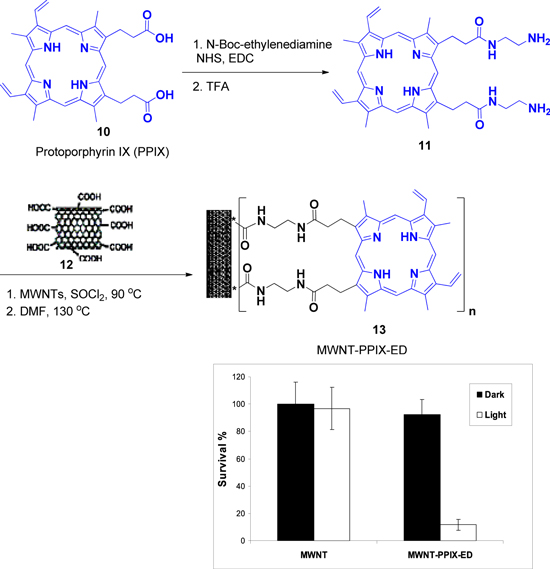
The application of modern nanotechnology in the development of photo-induced therapeutics was developed by D Mondal [70]. In that respect, the fascinating photodynamic property of porphyrin-CNT conjugate towards the application of antibacterial agents through a nanotechnology has been explored. To that purpose, a prolific consensus has emerged for conjugating porphyrin to a carbon nanotube to form nanocomposite films, as CNTs are emerging as attractive scaffolds for the immobilization of antimicrobial agents; CNT can be used to form ultrathin, flexible and transparent films.
In short, the carboxyl group of PPIX (protoporphyrin IX, 10) was coupled first with amino function of mono Boc-protected ethylenediamine (ED) in the presence of EDC and NHS followed by TFA treatment to get 11. Acid-functionalized multi walled carbon nanotubes (MWNTs) (12) were refluxed in thionyl chloride and then allowed to react with 11 to yield MWNT-PPIX conjugates (13) (figure 4). The MWNT-PPIX conjugates were dispersible in several solvents including water, DMF, and THF, following slight sonication for 2–5 min. Characterization by UV–vis spectroscopy further confirmed the attachment of PPIX to the MWNTs. Next, these were used for the antimicrobial assay through photoinduced toxicity of MWNT-PPIX conjugates to Gram-positive S. aureus cells. The conjugates are highly effective in small amounts and in short time scale.
Figure 4. Synthesis of MWNT-PPIX and plot showing the survival percentage of S. aureus treated with films containing acid-functionalized MWNT or MWNT-PPIX in the dark (black bars) and on irradiation with light for 1 h (white bars).
Download figure:
Standard image High-resolution imageAnother interesting research work was published by D Mondal [71] where they are not only concerned with the light activated antibacterial study of porphyrin conjugated CNT but have also extended their study for the effectiveness of this conjugated photosensitizer as antiviral agents. In this context, they used protoporphyrin IX (PPIX) (10), which was attached to acid-functionalized multi-walled carbon nanotubes (MWNTs, 11). The reason behind the use of carbon nanotubes as scaffolds was their ease of recovery from a solution through filtration. In the presence of visible light, MWNT-PPIX (13) was found to significantly reduce the ability of influenza A virus to infect mammalian cells. MWNT-PPIX (13) may be used effectively against influenza viruses with little or no chance of them developing resistance to the treatment. Furthermore, MWNT-PPIX (13) can be easily recovered through filtration, which offers a facile strategy to reuse the active porphyrin moiety to its fullest extent. Thus, MWNT-PPIX (13) conjugates represent a new approach for preparing ex vivo reusable antimicrobial and antiviral agents.
Carvalho et al [72] developed an exciting research work to combat microbial contamination of water or waste water. They have prepared cationic nanomagnet–porphyrin hybrids and deliberated their photodynamic therapeutic capabilities against the gram-negative E. coli bacteria, the Gram-positive Enterococcus faecalis (E. faecalis) bacteria and T4-like phage. The synthetic procedure is shown in figure 5 where the starting magnetic template is 14 and the porphyrins used in this work are 5-(pentafluorophenyl)-10,15,20-tris(4-pyridyl)porphyrin (15), 5-(pentafluorophenyl)-10,15,20-tris(1-methylpyridinium-4-yl)porphyrin triiodide (16) and 5-(pentafluorophenyl)-10,15,20-triphenylporphyrin (17). The grafting of porphyrins (15, 16, 17) on the magnetic nanomaterial 14 was carried out to obtain compounds 18–20 in DMSO at 140 °C and the reactions were monitored by TLC and UV–vis spectroscopy. Cationic nanomagnets were prepared by treating compounds 18–20 and 14 with CH3I in DMF at 40 °C to get 21, 22 and 23 which were characterized by x-ray, TEM, selected area electron diffraction (SAED) and solid state UV–vis spectra. The photostability of the nanomaterials 19, 21 and 22 were evaluated under the same conditions used in the biological assays (white light, 380–700 nm, 40 W m−2) (table 1).
Figure 5. Synthesis of the nanomagnet–porphyrin hybrids and the cationic nanomaterial.
Download figure:
Standard image High-resolution imageThe potentiality of the new hybrids was tested against E. faecalis, E. coli and T4-like phage. Surprisingly, hybrid 21 turned out to be the most effective nanomaterial, which at 20 μM caused a total photo-inactivation (to the limit of detection) of E. faecalis, E. coli, and T4-like phage, upon irradiation with white light of 21.6, 43.2, and 14.4 J cm−2, respectively. The results showed that these new multi charged nanomagnet–porphyrin hybrids are very stable in water and highly effective in the photo inactivation of bacteria and phages. Their remarkable antimicrobial activity, associated with their easy recovery, just by applying a magnetic field, makes these materials novel photosensitizers for water or waste water disinfection.
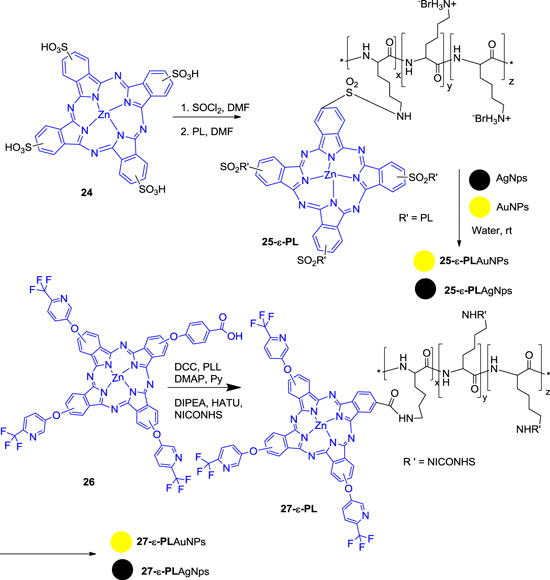
Nykong et al [73] explored the effectiveness of PC derivatives for the photodynamic antimicrobial chemotherapy (PACT). It is well known that PC derivatives act as PDT agents with high absorption coefficient (μ > 105 M−1 cm−1) in the phototherapeutic window (600–800 nm) together with a long triplet lifetime to generate cytotoxic singlet oxygen (1O2). In this report, they synthesized the zinc phthalocyanine (ZnPC)-ε-polylysine (ε-PL) conjugates as photodynamic sensitizers for the inactivation of Staphylococcus aureus (S. aureus). The selection of this bacterium was for its permeable outer membrane that allows for the diffusion of agents, as a defence mechanism. Such bacteria tend to develop resistance to antimicrobial agents.
The conjugation of nPC(SO3)4 (24) with polylysine ε-PL results in the formation of 25-ε-PL, wherein HPLC confirmed the presence of PC in the conjugation. Nanoparticle conjugations 25-ε-PL-AgNP, 25-ε-PL-AuNP and 27-ε-PL-AgNP, 27-ε-PL-AuNP were achieved by stirring a solution of either Au or Ag nanoparticle in the presence of complex 25-ε-PL and 27-ε-PL (figure 6).
Figure 6. Synthesis of Au and Ag nanoconjugate 25-ε-PL and 27-ε-PL.
Download figure:
Standard image High-resolution imageNext, 4-tetrakis-(5-trifluoromethyl-2-pyridyloxy) phthalocyaninato zinc(II), 26 and then conjugates with ε-PL to provide 27-ε-PL. The 5-trifluoromethyl-2-pyridyloxy substituents of complex 26 were chosen for solubility and to prevent aggregation. The photophysical and photochemical properties of the nanoparticles were studied along with their biological activities against different microorganisms. The minimum inhibitory concentration (MIC) against S. aureus is shown in table 2. The inactivation of S. aureus is compared to that of 25- ε -PL. The assay of minimum inhibitory concentration of the conjugates against S. aureus shows that the presence of silver nanoparticles greatly decreases the growth of the microorganism for both 25-ε-PL and 27-ε-PL conjugates and also it was observed that 27-ε-PL-AgNP is a more effective photosensitizer compared to 25-ε-PL-AgNP in the photodynamic antimicrobial chemotherapy against S. aureus at 39.6 mW cm−2. It was proposed that 27-ε-PL might have proper adhesion (due to the presence of polylysine) to the cell membrane that is sufficient for light-activated destruction of the bacterium.
Table 1. PS minimum inhibitory concentration (MIC) (μM) versus light dose (J cm−2). Causing the highest decrease in the survival (log) of the selected microorganism upon white light irradiation at a fluence rate of 40 W m−2.
| E. colia | T4-like phageb | |||||
|---|---|---|---|---|---|---|
| PS | [PS] (μM) | Light Dose (J cm−2) | Survival decrease (log) | [PS] (μM) | Light Dose (J cm−2) | Survival decrease (log) |
| 2 | 5 | 21.6 | 4.8c | 5 | 43.6 | 6.9c |
| 6 | 200 | 21.6 | 4.7c | 20 | 64.8 | 6.9c |
| 8 | 20 | 43.2 | 4.8c | 20 | 14.4 | 6.8c |
| 9 | 200 | 64.8 | 0.2c | 100 | 64.8 | 5.2c |
aInitial concentration = 105 CFU mL−1. bInitial concentration = 107 PFU mL−1. cDetection limit.
Table 2. S. aureus photo inhibition at fluence of 39.6 mW cm−2 for 10 min irradiation time at MIC50 conc. For 25-ε-PL and 29-ε-PL in pH 7.4.
| Compound | MIC50 (μM) dark | MIC50 (μM) light | MIC50 (μM) with AuNPs | MIC50 (μM) with AgNPs |
|---|---|---|---|---|
| 25-ε-PL | 0.75 | 0.187 | 0.046 | 0.0058 |
| 29-ε-PL | 0.375 | 0.046 | <0.046 | <0.0058 |
Recently, the same research group reported [74] the related work using phthalocyanine-based nanoparticle as photosensitizer for the antibacterial activity. For that rationale, the silver and gold nanoparticles were synthesized in different sizes and then conjugated to metallophthalocyanines with the central metal atom Cu, Ti, Sn and Zn inside the PC ring. (OH)2GeMCsPc, SnMCsPc, ZnMCsPc (MCsPc is monocysteinyl phthalocyanine) and OTiMCPc (MCPc is monocarboxy phthalocyanine) were prepared wherein the monocysteinyl moiety consists of both a carboxylic acid group and an amino group that can coordinate onto the surfaces of the silver and gold nanoparticles, hence protecting the latter against oxidation, whereas the rest of the peripheral positions contain phenoxy groups that allow solubility in most common organic solvents. Various structures such as spherical, triangular and clustered cubics were characterized by TEM, XRD and UV-visible spectroscopy. The different AgNPs were allowed to interact with different MPC complexes in DMF, as shown in figure 7, to obtain the phthalocyanine conjugated nanoparticles 29a-d. All the AgNPs were agglomerated in the presence of phthalocyanines 28a–d (figure 7), for spherical, triangular and cubic, respectively. The cubic AgNPs, in particular, were agglomerated and amorphous in the presence of phthalocyanines resulting in a difficulty to observe edges of the AgNPs.
Figure 7. Preparation of phthalocyanine-based nanoparticle as photosensitizer.
Download figure:
Standard image High-resolution imageThe antimicrobial activities of the AgNPs were tested without photosensitizers both in the presence of light and in the dark. The highest antimicrobial activity was achieved for both conjugates against Bacillus subtilis (B. subtilis) and compared to S. aureaus both in the dark and under illumination with light. The antimicrobial activity of the (OH)2GeMCsPC gave the highest production of singlet oxygen both alone and in the presence of AgNPs. The spherically shaped AgNPs showed the best antibacterial activity followed by the triangular shaped AgNPs when compared in the absence of MPC complexes. The antimicrobial activity of the AgNPs alone can be expressed in an increasing order as follows: cubic < triangular < spherical. The antimicrobial activities of various MPCs can be expressed as SnMCsPC < OTiMCsPC < ZnMCsPC < (OH)2GeMCsPC and the antibacterial activity for the combinations of MPCs with various AgNPs showed the same order as MPCs alone (figure 8).
Figure 8. Bar graph representation of antimicrobial activities of various AgNPs shapes towards S. aureus in the dark and illuminated with visible light (a). Antimicrobial activities of (OH)2GeMCsPc–AgNPs towards S. aureus at various concentrations in the dark and under illumination with light (b).
Download figure:
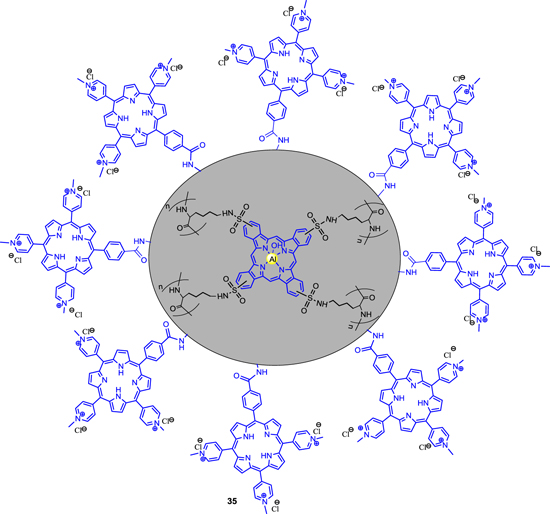
Standard image High-resolution imageKuruppuarachchi et al [75] designed and synthesized polyacrylamide nanoparticles as a delivery system in photodynamic therapy. To that objective, synthesized porphyrin-acrylamide (PCNP-A) (in suspension) was mixed with synthetic porphyrin bearing a single amine-reactive succinimide-N-oxycarbonyl-phenyl group, 5-[4-(succinimide-N-oxycarbonyl)-phenyl]-10,15,20-tri(4-N-methylpyridiniumyl porphyrin) in water to produce polylysine bound tetrasulfonato-aluminum phthalocyanine entrapped nanoparticles coated with a second, porphyrin based, photosensitizer (PCNP-P) which was confirmed by clear absorbance peaks at 424 nm (porphyrin, Soret band) and 680 nm (PC, Q-band), respectively. As confirmed by the PCS and TEM images, the mean size of the PCNP-P was 95 nm ± 10 nm (figure 9). However, the polyacrylamide nanoparticles have potential as delivery vehicles for photodynamic agents in photodynamic therapy for cancer but also the cationic nature of PCNP-P makes it potentially attractive for photodynamic antimicrobial chemotherapy (PACT).
Figure 9. Polymer bound tetrasulfonato-aluminium phthalocyanine entrapped porphyrin conjugated nanoparticles.
Download figure:
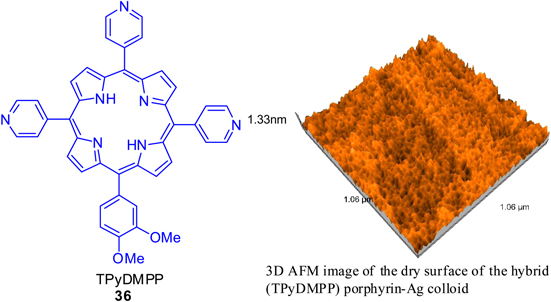
Standard image High-resolution imageIn 2013 Creanga et al [76] published another application of PACT by producing spherical and ovoid disk-shaped porphyrin-silver colloid hybrid nanoparticles with sizes in the range of 30–49 nm and 65–120 nm for wide-band absorption. These particles were characterized by UV–vis, fluorescence and atomic force microscopy (AFM). The preparation of hybrid silver colloid-A3B porphyrin complex was performed by electrostatic interactions between negatively charged Ag-colloid to the positively charged A3B porphyrins, namely, 5,10,15-tris-(4-pyridyl)-20-(3,4-dimethoxy-phenyl)-porphyrin (TPyDMPP) (36) in acidic medium by continuous addition of porphyrin derivative and HCl in small portions (figure 10). The optical absorbance of hybrid silver colloid-porphyrin system in the range of 320–770 nm recommend them as one of the potential materials in sensors design. In addition, it could find applications as antimicrobial and photosensitizers in PDT and photovoltaic cell.
Figure 10. 5,10,15-tris-(4-pyridyl)-20-(3,4-dimethoxy-phenyl)-porphyrin (TPyDMPP) (36).
Download figure:
Standard image High-resolution imageIt is noteworthy to mention that cationic amphiphilic cyclodextrins are being used as supramolecular aggregates in drug delivery. In this regards, Ferro et al [77] have shown the usage of cyclodextrins in the field of PDT as nanoaggregates and photosensitizers for antimicrobial (figure 11). For these purposes, TDPyP/cyclodextrin was prepared by adding a solution of TDPyP (37) in ethanol and a solution of the SC6NH2 (cyclodextrin) (38) in CHCl3 and the resulting complex was examined for photosensitizing efficiency against methicillin-resistant S. aureus (MRSA) and E. coli. The hybrid was further investigated by dynamic light scattering (DLS), zeta potential measurements, and TEM after negative staining with uranyl acetate. It has been observed that the photobacterecidal activity is enhanced after the introduction of porphyrin unit.
Figure 11. Chemical structures of the investigated photosensitizer and amphiphilic cyclodextrin: 5-[4-(1-dodecanoylpyridinium)]-10,15,20-triphenyl-porphyrinyl chloride (TDPyP) (37) and heptakis (2-ω-amino-O-oligo(ethylene oxide(-6-hexylthio)-β-cyclodextrin (SC6NH2) (38).
Download figure:
Standard image High-resolution imageFigure 12. Zinc phthalocyanine to silver and gold nanoparticles.
Download figure:
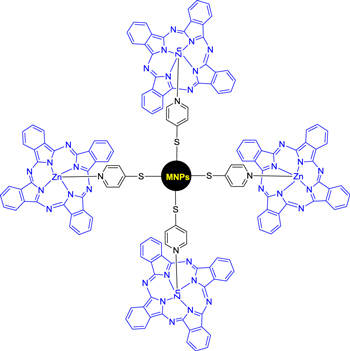
Standard image High-resolution imageMasilela et al [78] prepared zinc phthalocyanine and bis-(1,6-hexanedithiol) silicon phthalocyanine to silver and gold nanoparticles for antibacterial activity (figure 11). After conjugation of the phthalocyanine with the metal nanoparticles, it shows red shifting of absorption spectra and also there is an improvement in the photophysico chemical behaviour and antimicrobial activity for both complexes. For this phthalocyanine metal nanoparticle conjugates triplet lifetimes were decreased. The Zn phthalocyanine complex gave the highest triplet and singlet oxygen quantum yield in the presence of gold nanoparticles. The bacterial inhibition was observed to be best for the Si phthalocyanine derivative in the presence of nanoparticles in comparison to the Zn phthalocyanine counterpart. The highest antimicrobial activity was reached for both conjugates against B. subtilis compared to S. aureaus both in the dark and under illumination with light.
5. Conclusions
This review focuses on the recent developments in the synthesis of porphyrin and phthalocyanine based light-triggered nanotherapeutics as a useful tool for antimicrobial applications. At this moment, the literature presents several options of photosensitizers (PS) drugs available for PDT and the associations of these PS with different nanoparticulated carriers have been shown as a suitable strategy to improve some photophysical and antimicrobial features related to the therapy. There is a decrease in the risk of induction of resistant microbial species against PDT when these PS drugs are associated with nanoparticulated carriers. This review describes the recent research on the porphyrins and phthalocyanines incorporated nanoparticles through covalent linkage or through physical absorption and their activities against microorganisms.