Abstract
In this paper, CoFe2O4/chitosan-graft-poly(acrylic acid) (CoFe2O4/CS-graft-PAA) nanocomposites were prepared successfully by coprecipitation of the compounds in alkaline solution and were used for removal of nickel (II) ions from aqueous solution. The sorption rate was affected significantly by the initial concentration of the solution, sorbent amount, and pH value of the solution. Batch experiments were conducted to investigate the adsorption capacity under different initial concentration (ranging from 25 to 150 mg L−1), solution pH (4.1, 5.3, 6.4 and 7.6), and contact time. These nanocomposites can be recycled conveniently from water with the assistance of an external magnet because of their exceptional properties. The prepared nanocomposites were characterized by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), x-ray powder diffraction (XRD), and thermogravimetric analysis (TGA).
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The toxic heavy metals present in wastewater, effluents and soils are becoming an increasingly important issue because of growing economic and environmental concerns. One of the metals released to the environment is nickel from a number of sources such as electroplating processes, nickel batteries, alloys, and steels [1]. Several methods for removal of the heavy metals from wastewater have been reported including precipitation, filtration, ion exchange, biological process, chemical reactions, and adsorption [2, 3]. Among these, adsorption is considered as an effective and economical method for the removal of pollutants from wastewater due to ease of handling, and effective and economical treatment process. There are many materials used for adsorption of heavy metal such as active carbon, chitosan (CS) bead, betonies and so on. However, these materials are difficult or not able to be recycled after adsorption, if the adsorbent was prepared with a very small size for improvement of the adsorption efficiency by increase of the surface area. Therefore, it is interesting to improve the separability of the adsorbents having small size. Recently, attention has been paid to the magnetic separation technology. With the aid of magnetic force, magnetic matters can be separated from the water efficiently regardless of its size.
CS has also increasingly been studied as an adsorbent for the removal of heavy metal ions from aqueous solutions because the amino and hydroxyl groups on the CS chain act as chelation or reaction sites for the substances. These groups function as the coordination sites for heavy metal ions [4, 5]. Several studies of metal ion adsorption by CS have been carried out in recent years, such as the removal of copper [6], chromium [7], cadmium, nickel and lead ions from aqueous solution [3]. The aim of this study was to synthesize magnetic chitosan-graft-poly(acrylic acid) composite (CoFe2O4/CS-graft-PAA) for removal of the heavy metal from aqueous solution. The sorption of CoFe2O4/CS-graft-PAA nanoparticles for nickel ion was also evaluated. The effect of various experimental conditions such as the initial concentration, sorbent amount, temperature and pH value of the solution on the adsorption capacity of the magnetic composite for nickel ions were investigated.
2. Materials and methods
2.1. Materials
CS with the deacetylation degree of 90% was prepared from our lab. Acrylic acid (AA) was obtained from Aldrich-Sigma Chem. Co. Potassium persulfate (K2S2O8) was recrystallized from distilled water, glutaraldehyde (GLA), iron (III) chloride hexahydrate (FeCl3.6H2O), cobalt (II) chloride hexahydrate (CoCl2.6H2O), sodium hydroxide (NaOH) were purchased from Aldrich-Sigma Chem. Co. All the other materials were reagent grade and used without further purification. Metal stock solutions were prepared (1000 ppm) by dissolving the Ni(NO3)2.6H2O metal salts in deionized distilled water. The pH of 4–6 and 8 of medium solutions was adjusted by adding acetate buffer solutions and ammonium chloride solution, respectively. Deionized distilled water was used in all experiments.
2.2. Preparation of CoFe2O4 magnetic nanoparticle
The synthesis of CoFe2O4 magnetic nanoparticles was prepared by the coprecipitation method and based on the mixture of FeCl3 · 6H2O and CoCl2 · 6H2O salts with the molar ratio of 2:1. Briefly, 1.84 g FeCl3 · 6H2O and 0.8 g CoCl2 · 6H2O were dissolved in 85 mL of deionized water. 2 M solution of NaOH was prepared and slowly added to the salt solution dropwise. The pH of the solution was constantly monitored by adding the NaOH solution under a magnetic stirrer until a pH level of 11–12 was reached. The precipitation immediately occurred to change the reaction solution to dark-black. Stirring of the resulting solution was continued for one hour and the product was cooled to room temperature. After that, the precipitation was magnetically separated by an external magnet, and the pH level was reduced to approximately 7–8 by washing with deionized water and then was washed with ethanol to remove the excess agents from the solution. Finally, CoFe2O4 magnetic nanoparticle was obtained.
2.3. Preparation of CoFe2O4/CS-graft-PAA nanocomposite
The CS-graft-PAA copolymer was obtained by polymerization of AA in CS solution. 4 g of CS flakes was dissolved in 400 ml of 1% (w/w) AA solution under mechanical stirring. Until the solution became clear, 1 mmol of K2S2O8 was added into the above-mentioned solution and stirring was continued at 60 °C for 1 h. Then, CoFe2O4 magnetic nanoparticle was added into the CS-graft-PAA solution and CoFe2O4/CS-graft-PAA nanocomposite was obtained by adding 2 M NaOH solution; final pH of the solution was 9–10 and the reaction was allowed to proceed at 60 °C for 2 h. At the end of polymerization, 4 mL of 25% GLA was added to the reaction system at 40 °C and cross-linking reaction was conducted for 1 h. The final product was washed with distilled water several times until pH reached neutral, then collected by magnetic separation and dried for further application.
2.4. Characterization
Scanning electron microscopy (SEM) images were taken with 7410F–JMS–JEOL electronic scanning microscope (Japan) to examine the morphology and surface structure of the adsorbent. The transmission Fourier transform infrared spectroscopy (FTIR) spectra were recorded with a Vector 22–Bruker (Germany) and the range of the scanning wave numbers was 500–4000 cm−1. The structural properties of synthesized nanoparticles were analyzed by x-ray powder diffraction (XRD) with a D8 Advance–Bruker System (Germany). Thermogravimetric analysis (TGA), thermal stability as well as the content of cobalt ferrite in the nanocomposites were analyzed by a thermogravimetric analyzer (Q500, Japan) in air atmosphere. The temperature ranged from 30 to 800 °C at a scanning rate of 10 °C min−1. Magnetic characterization of the nanoparticles was conducted using vibrating sample magnetometer (EV11-VSM, USA) at room temperature with a magnetic field in the range of −10 kOe to 10 kOe, where parameters such as saturation magnetization and coercive field were evaluated.
2.5. Batch adsorption studies
The adsorption of Ni(II) was analyzed in a batch system at room temperature with varied concentrations ranging from 25–50 mg L−1. The solutions were prepared in deionized distilled water using the Ni(NO3)2 · 6H2O. The stock solution was then diluted to give a standard solution of appropriate concentration.
2.5.1. Effect of initial concentration and contact time
In brief, 50 mL of metal ion solution was placed in a 100 mL beaker, and then 0.5 g CoFe2O4/CS-graft-PAA nanocomposite was added. The mixtures were then kept at room temperature. The aqueous samples were taken at various contact times: 0.5, 1, 2, 3, 4 and 5 h. The concentrations of Ni ions were measured using standard methods recommended for examination of water and wastewater [8]. The amount of adsorption at time t, qt
(mg g−1), is calculated by  , where C0 and Ct
(mg L−1) are the liquid-phase concentrations of Ni ions at initial and any time t, respectively; V is the volume of the solution (L); W is the mass of used dry adsorbent (g). The percentage adsorption was determined by
, where C0 and Ct
(mg L−1) are the liquid-phase concentrations of Ni ions at initial and any time t, respectively; V is the volume of the solution (L); W is the mass of used dry adsorbent (g). The percentage adsorption was determined by  , where
, where  and
and  are the initial and equilibrium concentration of metal ion solution (mg L−1).
are the initial and equilibrium concentration of metal ion solution (mg L−1).
2.5.2. Effect of pH
The effect of the pH of the suspending medium on metal removal was studied by performing equilibrium sorption experiments at different pH values. The range of pH was 4.1–7.6 to eliminate the consequences of precipitation of the metals as hydroxides at higher pH. The initial concentration of metal ions in each aqueous solution was 50 mg L−1 and 0.5 g CoFe2O4/CS-graft-PAA were weighed and immersed into absorbed solutions with different initial pH under continuous shaking at room temperature. The contact times were varied from 0.5, 1, 2, 3, 4 and 5 h.
2.5.3. Effect of adsorbent dosage
Initial concentration of 50 mg L−1 of Ni(II) solutions were used for this experiment. 50 mL of metal ions solution was added into the Erlenmeyer flasks containing 0.1 g, 0.2 g, 0.25 g and 0.5 g absorbent, respectively. The mixtures were continuously stirred at room temperature and optimum pH of metal ions solution at pH 5.3. The contact times of these experiments were varied from 0.25, 0.5, 1, 2 and 3 h.
2.5.4. Effect of temperature
The reaction was carried out in a controlled temperature water bath at different temperature conditions in the range of 30–50 °C. During these experiments the metal concentration (50 mg L−1), and the amount of CoFe2O4/CS-graft-PAA (0.5 g) were kept constant.
3. Results and discussion
3.1. Characterization of CoFe2O4/CS-PAA nanocomposites
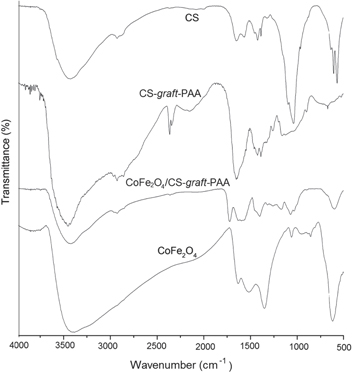
The preparation of CoFe2O4/CS-graft-PAA nanocomposites was conducted through coprecipitation of the mixture containing CS-graft-PAA copolymer and ferric compounds in alkaline solution were described in the experimental part. First, when CS is added into AA aqueous solution, CS–AA salts will be formed through the strong electrostatic interaction between –NH2 of CS and –COOH of AA, and then, the polymerization of AA was initiated by K2S2O8 [9]. When the polymerization of AA reached a certain level, the inter- and intra-molecular linkages occurred between carboxyl groups from PAA and positively charged amino groups of CS. These linkages could make the macromolecular chains of CS roll up, which was responsible for the formation of the gelation of the CS solution. As the polymerization time extended, the amount of PAA in the solution increased, the CoFe2O4/CS-graft-PAA nanocomposite was formed when CoFe2O4 magnetic fluid was added into CS-graft-PAA solution. FTIR spectra of CoFe2O4 nanoparticles, CS, CS-graft-PAA and CoFe2O4/CS-graft-PAA nanocomposites are shown in figure 1. Absorption peak at 617 cm−1 is assigned to Fe–O group in CoFe2O4. For the spectrum of CS, the characteristic absorption bands appeared at around 3449 cm−1 corresponding to the stretching vibration of O–H and N–H bonds. The peaks at 2923 cm−1, 1641 cm−1, 1388 cm−1, 1029 cm−1 are ascribed to C–H of alkyl group, C=O of amide I, -NHCO of amide III and C–OH bond, respectively.
Figure 1. FTIR spectra of CoFe2O4 nanoparticles, CS, CS-graft-PAA, CoFe2O4/CS-graft-PAA nanocomposite.
Download figure:
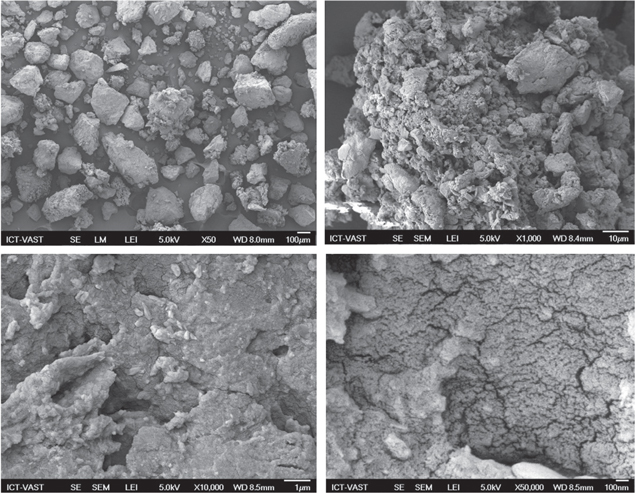
Standard image High-resolution imageThe surface morphology of nanocomposite is described in figure 2. The result illustrates the surface texture and porosity of CoFe2O4/CS-graft-PAA nanocomposite with holes and small openings on the surface, thereby increasing the contact area, which facilitates the pore diffusion during adsorption [10].
Figure 2. Scanning electron microscopy of CoFe2O4/CS-PAA nanocomposites.
Download figure:
Standard image High-resolution imageXRD is also used to examine the crystallite structure of CoFe2O4 nanoparticles, CS and CoFe2O4/CS-graft-PAA nanocomposites. The pattern of pure CoFe2O4 is shown in figure 3, the main peaks are at 2θ values of 30°, 35.8°, 43.2°, 57° and 62.9°. These peaks match well with face-centered cubic cobalt ferrite. These results are consistent with the previous results [11, 12]. The XRD patterns of the CS exhibit two characteristic peaks at 2θ values of 11.3° and 20.2°. The peak at 20.2° shows the allomorphic tendon form of CS, which resulted in a strong decrease in the sorption capacities. Additionally, XRD parttern of CoFe2O4/CS-graft-PAA nanocomposite shows the mixed peaks of CS and the pure CoFe2O4 nanoparticles. However, the diffraction intensity of CoFe2O4/CS-graft-PAA nanoparticles is lower than that of CoFe2O4 due to lower CoFe2O4 content. This result suggests that the CoFe2O4 nanoparticles are incorporated into CS-graft-PAA and the structure of CoFe2O4 nanoparticles is not changed during the formation of CoFe2O4/CS-graft-PAA composite particles.
Figure 3. XRD of Chitosan (a), CoFe2O4 nanoparticles (b) and CoFe2O4/CS-PAA composite particles (c).
Download figure:
Standard image High-resolution imageThe magnetization curves of the naked CoFe2O4 nanoparticles and CoFe2O4/CS-PAA nanocomposite are illustrated in figure 4, which shows that the magnetization of the samples would approach the saturation values when the applied magnetic field increases to 10 000 Oe. The saturation magnetization of naked CoFe2O4 nanoparticles is 16.579 emu g−1. For the CoFe2O4/CS-graft-PAA nanocomposite, the saturation magnetization is about 2.632 emu g−1. The loss of magnetization was attributed to the non-magnetization organic components, which reduced the total magnetization [13]. The composite particles show strong magnetic properties and can be separated easily from the solution with the help of an external magnetic force. A narrow hysteresis loop can be seen in figure 4. There is a small remnant magnetization. It could be explained that some particles are magnetically blocked.
Figure 4. The magnetization curve of CoFe2O4 nanoparticles and CoFe2O4/CS-graft-PAA composite particles at room temperature.
Download figure:
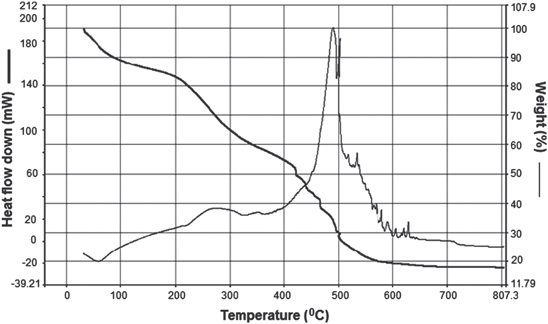
Standard image High-resolution imageAccording to TGA analysis, the content of CoFe2O4 nanoparticles in the CoFe2O4/CS-PAA nanocomposites is about 17.7% (figure 5). There are three stage weight losses in the temperature ranges from 40 to 800 °C. First weight loss is about 11.1% ranged 40–100 °C, which corresponds to the adsorbed and bound water in the sample. The second weight loss is about 32.3% in the range from 100–380 °C, which was ascribed to the decomposition of CS and the carboxyl groups of PAA. Moreover, the weight loss of 38.9% in the range from 380 to 800 °C is due to the breakage of PAA chain and the dehydroxylation of CoFe2O4 upon thermal treatment [14].
Figure 5. TGA curve of CoFe2O4/CS-PAA nanocomposites.
Download figure:
Standard image High-resolution image3.2. Batch adsorption experiment
3.2.1. Effect of initial concentration and contact time
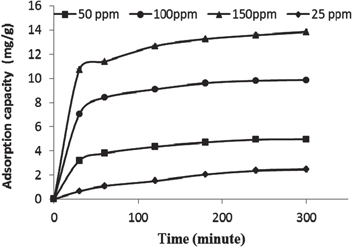
The adsorption capacity depends on the initial concentration and the contact time. As shown in figure 6, it is remarkable that an increase in the initial concentration (25–150 ppm) for Ni(II) ions led to an increase in the adsorption capacity of CoFe2O4/CS-graft-PAA nanocomposite at various contact times. Other parameters such as dose of the adsorbent, pH of the solution and agitation speed were kept constant. This can be attributed to the mass transfer effects and the driving force of the concentration gradient being directly proportional to the initial concentrations. The plateau values indicated that the adsorption equilibrium was gradually attained. The equilibrium adsorption capacity of Ni(II) ions was 2.46, 4.96, 9.86 and 13.84 mg g−1 CoFe2O4/CS-graft-PAA for 25, 50, 100 and 150 ppm, respectively. The sorption percentage was higher than 98% with concentration of 100 ppm and decreased to 92.3% at 150 ppm. It appears that as adsorbate concentration was raised, binding capacity of the adsorbent reached instantaneous saturation resulting in diminishing the overall % adsorption of Ni ions.
Figure 6. Effect of initial concentration and contact time on equilibrium adsorption of Ni(II) ions onto CoFe2O4/CS-graft-PAA.
Download figure:
Standard image High-resolution image3.2.2. Effect of pH solution
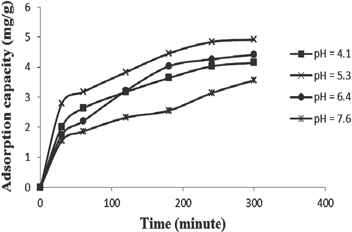
The pH of the aqueous solution is an important variable, which controls the adsorption of the metals on the solid–water interfaces. The pH affects the availability of metal ions in solution and the metal binding sites of the adsorbent. The obtained CoFe2O4/CS-graft-PAA particles were incubated in buffer solution with different pH values. Results of these experiments showed that these nanocomposites were stable in distilled water and acidic media in a range of pH values from 4.1 to 7.6. However, the concentration of OH− is high when pH is higher than 7.6, and OH− reacts with Ni2+ to form the corresponding precipitations, which causes the decline of absorption capacity. The result is graphically represented in figure 7. The maximum adsorption capacity of Ni(II) ions occurred at pH 5.3. In addition, the maximal Ni(II) uptakes of CoFe2O4/CS-graft-PAA was 4.92 (mg g−1) and the amount of sorption percentage was 99.2%. After combination of PAA and CS, the resultant composite could have not only improved Ni(II) uptake but also good biodegradability. Overall, CoFe2O4/CS-graft-PAA could be considered as a promising adsorbent for removal of metal ions for high adsorption capacity, good biodegradability and rapid separation ability from aqueous solutions.
Figure 7. Effect of pH on adsorption of Ni(II) on CoFe2O4/CS-graft-PAA nanocomposites.
Download figure:
Standard image High-resolution image3.2.3. Effect of adsorbent dosage
The dependence of metal ion sorption on dose was studied by varying the amount of adsorbents from 0.1 g to 0.5 g, while keeping the volume (50 mL) and concentration (50 ppm) of the solution constant at room temperature and corresponding to optimal pH of solution. The results are graphically represented in figure 8.
Figure 8. Effect of dose and contact time of CoFe2O4/chitosan poly(acrylic acid) nanocomposites on percentage removal of Ni(II) ion.
Download figure:
Standard image High-resolution imageIt can be seen that the rate of removal of Ni ions increases with the increase in the dose of adsorbent. The percentages of removal increased from 93 to 95% with dose from 0.1 g to 0.5 g, respectively. There was a substantial increase in adsorption when the dose of adsorbents was increased from 0.1 g to 0.5 g. This was expected due to the fact of the higher the dose of adsorbents in the solution, the greater the availability of exchangeable sites for the ions.
3.2.4. Effect of temperature on absorption of CoFe2O4/CS-graft-PAA
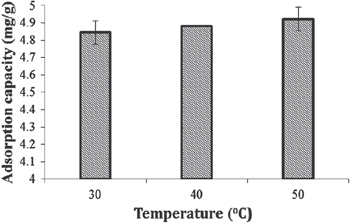
It was observed that temperature had a pivotal role in the complex of metal ion and CS derivative. As shown in figure 9, the absorption of metal ions appeared to increase as the temperature increased to 50 °C. The absorption capacity increases from 4.62 to 4.88 mg g−1. It could be explained that the absorbent molecular and absorbed ions move much faster at the high temperature, the interactions among molecules decrease, and the metal ions can more easily move into absorbent. As a result, the contact area between absorbent and metal ions increases, which leads to the increase of the absorption capacity of absorbent for metal ions. On the other hand, the absorption here is an endothermic process, therefore, the rise of temperature promotes the absorption of absorbent for metal ions; the temperature has to be optimum, preferably at 50 °C.
Figure 9. Effect of temperature on absorption of CoFe2O4/CS-graft-PAA nanocomposites for Ni(II).
Download figure:
Standard image High-resolution image4. Conclusions
Magnetic CS-graft-PAA nanocomposite with a high CoFe2O4 loading content have been readily prepared by polymerization of AA with CS. The magnetic composite was characterized by FTIR, XRD, SEM, TGA and VSM. This study has evidenced the improved adsorption behavior of CoFe2O4/CS-graft-PAA to remove nickel ions from water. In addition, the magnetic composite adsorbents also showed superparamagnetic property and significant improvement of the separability from aqueous solutions.