Abstract
This work presents the results of the successful preparation of Pd nanoparticles by the polyol method and the proposed techniques of controlling their size and shape. Polyvinylpyrrolidone (PVP) stabilized Pd nanoparticles of various shapes with the largest sizes in the forms of octahedrons (24 nm), tetrahedrons (22 nm) and cubes (20 nm) have been obtained by alcohol reduction in ethanol with the addition of a hydrochloric acid catalyst. Moreover, PVP–Pd nanoparticles of well-controlled spherical shapes have also been prepared by a modified polyol method. PVP–Pd nanoparticles of cubic, octahedral, tetrahedral and spherical shapes with well-controlled size achieved by using ethylene glycol (EG) as reductant and various inorganic species were also fabricated. In particular, Pd nanorods with sizes of 47 nm and 16 nm formed due to the anisotropic growth mechanism of Pd nanoparticles were found. At the same time, tetrahedral particles of sharp shapes of 120 nm and 70 nm sizes have been observed. A high concentration of inorganic species was used to control the size and shape of the Pd nanoparticles, leading to the appearance of various irregular sizes and shapes. There was evidence of the very sharp corners and edges of tetrahedral and octahedral Pd nanoparticles or others that were formed in the clustering and combination of the seeds of smaller particles.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Recently, Pd nanoparticles have been studied and used as very efficient catalysts for the catalytic reactions of various compounds [1–8]. The catalytic activity is dependent on both their size and shape. Pd nanoparticles of very sharp shapes exhibit strong catalytic activity for the organic reactions of Suzuki, Heck and Stille couplings [3, 4]. They have retained their catalytic properties in an aqueous suspension, which makes them ideal for studying the size dependence of the catalytic behavior [5, 6]. At present, the issues of controlling the size and shape of Pd nanoparticles by the polyol method have been studied in the synthetic procedures for their potential applications as catalysts. In addition, the polyol method has been used to fabricate Pt nanoparticles with their well-controlled size and shape [9–11]. Moreover, it has been shown that single, bimetal and supported nanostructures directly related to Pd nanoparticles have been exploited in their potential applications as catalysts [12–18]. In this work, we prepared Pd nanoparticles by the polyol method with the addition of AgNO 3, and performed the control of their size and shape according to the rate of precursors, temperature and time of synthetic reactions at 160 o C. The increase in the content of silver nitrate up to 0.06 M AgNO 3 led to the appearance of a lot of bigger porous Pd nanoparticles with their spherical or quasi-spherical shapes. Here we observed that a low content of AgNO 3 should be used in the synthesis of Pd nanoparticles, which possibly makes the most of the Pd nanoparticles to have well-controlled sizes and shapes for their potential applications in catalysis.
2. Experimental procedure
2.1. Materials
The salts of PdCl 2 and Na 2 PdCl 4 were used as good precursors. In addition, AgNO 3 ,FeCl 3 and NaI were possibly used as efficient agents for the size and shape control. EG was used as both a reductant and a solvent at 160 o C. Polyvinylpyrrolidone (PVP, 50 000) was used as a stabilizer. Other chemicals used were methanol, ethanol, hexane (Aldrich), Aqua Regia and HCl (acid 37%). Distilled water and deionized water from the Narnstead nanopure H 2 O purification system were used to wash all experimental glassware.
2.1.1. PVP–Pd nanoparticles: various sizes and shapes
Typically, 0.1780 g of PdCl 2, 12 ml of 0.2 M HCl and 500 ml of distilled water were mixed to get H 2 PdCl 4 solution with a concentration of 2 mM. Then, the total volume was put in a flask, refluxed for 3 h and allowed to age for 2 days. The color of the product was typically pale-yellow. Then, 30 ml of 2 mM H 2 PdCl 4 was used with the addition of 40 ml of deionized water, 0.1334 g of PVP and 8 drops of 1 M HCl. Then, the mixture was heated at 120 o C. 28 ml of ethanol was added for the reduction of H 2 PdCl 4. The solution was refluxed for 3 h, resulting in a dark brown solution. Thus, the reduction of H 2 PdCl 4 by ethanol (as both solvent and reducing agent) occurred. Consequently, Pd nanoparticles were formed and stabilized by PVP. To evaluate their characterization, various volumes of Pd solution were centrifuged at 15 000 rpm for 30 min by a Sigma 3K30C-Kubota centrifuge, separated and precipitated by adding a triple volume of acetone. Then, the mixture was centrifuged and redispersed in 3 ml of ethanol by an ultrasound generator at 200 W, 37 kHz for the generation of Pd nanoparticles. To wash Pd nanoparticles, 9 ml of hexane was added and the solution was centrifuged at 15 000 rpm for 5 min. The product was washed three times with ethanol and hexane.
2.1.2. Synthesis of PVP–Pd nanoparticles: spherical shapes
In this process, 5 ml of EG was put in a volumetric flask, heated at 160 o C and stirred for 2 h. Then, 0.0162 g of Na 2 PdCl 4 was dissolved in 1.5 ml of EG under stirring for 2 h and the color of the mixture was typically red–brown. Then, 0.0312 g of PVP was dissolved in 3 ml of EG and stirred for 2 h. In a typical 50 times process, the contents of the solutions of PVP and Pd salt were injected into the flask every 30 s per one time (for example, 30 μl of Na 2 PdCl 4 solution, 60 μl of PVP) and the reaction continued for 5 min. Pd nanoparticles with the dark-brown color were formed with the stabilization by PVP. Then, the procedures of the centrifugations and washes of Pd nanoparticles were carried out and repeated until the Pd nanoparticles were dispersed in ethanol.
2.1.3. Synthesis of PVP–Pd nanoparticles: cubic, octahedral, spherical and various shapes
A volume of 5 ml of EG in a volumetric flask was heated at 160 o C and stirred for 2 h. Then, 0.0162 g of Na 2 PdCl 4 was dissolved in 3 ml of EG at room temperature under stirring for 2 h and the color of the mixture was typically red-brown. 0.0312 g of PVP was dissolved in 1.5 ml of EG at room temperature with a molar ratio of PVP/Pd salt equal to 12 : 1. The mixture was stirred for 2 h. Then, by the addition of AgNO 3, 0.5 ml of 0.0002 M AgNO 3 (similarly to 0.5 ml of 0.002, 0.02, and 0.06 M AgNO 3) was put into the flask and the mixture was continuously stirred for 2 h. Then, the solutions of PVP and Pd salt were injected into the flask. In a typical 50 times process, the contents of PVP and Pd precursor were injected into the flask at 160 o C every 30 s per one time (for example, 30 μl of Na 2 PdCl 4, 60 μl of PVP). The reaction continued for 5 min to obtain one sample and for 30 min for another sample. As a result, the chemical reduction of Na 2 PdCl 4 by EG occurred and Pd nanoparticles were formed with the stabilization by PVP. Similarly, the cases of the additions of 0.02 M FeCl 3, 0.06 M FeCl 3 or 0.02 M NaI were done so that the stepwise procedures were repeated until the Pd nanoparticles were dispersed in the ethanol.
2.2. Measurements for characterization
The drops of 10 μl of colloidal Pd solution were placed onto a carbon-film-coated copper grid and allowed to dry for 6 h. Some small volumes of the reaction mixture were studied by UV-vis-NIR spectroscopy using a Ubest 570 spectrotometer. The formation of products of Pd nanoparticles was proved by UV-vis spectroscopy. To characterize the Pd nanoparticles, the drops of solution containing colloidal Pd nanoparticles were set onto a copper grid. After that, the images of the Pd nanoparticles were obtained by a JEOL-JEM-2010XII microscope operated at 200 kV with a magnification of up to 1 500 000 times. In addition, the TEM data of the particle shape, size and area electron diffraction of the Pd nanoparticles were also analyzed.
3. Results and discussion
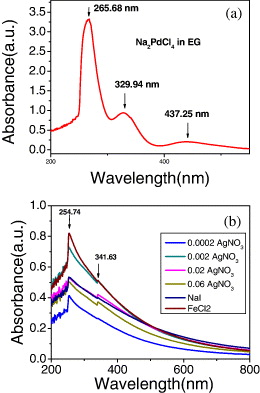
Figure 1 shows the UV-vis spectra of Na 2 PdCl 4 in EG with the strong peak at 265 nm and two peaks much weaker at 329.94 nm and 437.25 nm. In the case of PVP-stabilized Pd nanoparticles with the addition of very small amounts of silver nitrate of the concentration changing from 0.002 to 0.06 M AgNO 3, as well as the addition of small amounts of NaI and FeCl 3, the results showed a sharp and strong peak at 254.74 and a weak peak in the 341.63 nm range due to the ligand-to-metal charge-transfer transition of Na 2 PdCl 4 leading to Pd nanoparticles being stabilized by the PVP. In this process, Pd nanoparticles with the size in the range of 2–30 nm were obtained from the aqueous solution of PdCl 2 in the presence of PVP.
Figure 1 UV-vis spectra of (a) Na 2 PdCl 4 in ethylene glycol and (b) PVP-protected Pd nanoparticles in the range of 268–270 nm due to the ligand-to-metal charge-transfer transition of [PdCl 6]−2 ions of N and/or O atoms of PVP to Pd 4+ ion.
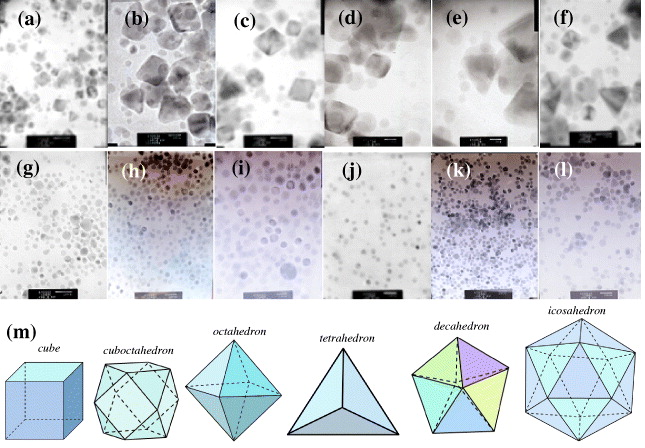
In figures 2(a)–(f), various shapes of Pd nanoparticles are spheres, cubes, tetrahedrons, octahedrons, twinned nanoparticles of twinned decahedral and icosahedral shapes, and other irregular shapes that are characterized by the formation of Pd nanoparticles by alcohol reduction [13]. The size of the biggest Pd nanoparticles is ∼24 nm. They have the shape of an octahedron of {111} facets. The biggest size of tetrahedral Pd nanoparticles is 22 nm. Cubic Pd nanoparticles of {100} facets with their biggest size of 20 nm are very clean. Many spherical minor Pd nanoparticles were observed by this method. Importantly, most of the large Pd nanoparticles with tetrahedral, octahedral and cubic shapes are formed due to the aggregation of the smaller seeds of spherical Pd nanoparticles, as shown in figures 2(a)–(f). Thus, EG plays the role of a structure-directing agent for the Pd nanoparticles. However, these Pd nanoparticles are still truncated at their sharp corners and edges, and the spherical shapes of the Pd nanoparticles are controlled by adjusting the rate of the Pd precursor and the PVP. The distribution of spherical Pd nanoparticles is homogeneous in figures 2(g)–(i). To control their size, 0.5 ml of 0.002 M AgNO 3 is added in the solutions prior to the addition of PVP and Na 2 PdCl 4 that has led to Pd nanoparticles of the controlled size less than 6 nm. Figure 2(j) shows the good size distribution of Pd nanoparticles despite various shapes, such as spherical, cubic, cuboctahedral, octahedral and tetrahedral, because the mixture was kept in the flask at 160 o for 5 min. However, size distribution is controlled by the addition of 0.02 M AgNO 3 in figure 2(j). The particle size distribution in figure 2(j) was very good. There are various sizes and shapes in figures 2(k) and (l).
Figure 2 TEM images of major sphere shapes and various sizes of Pd nanoparticles. TEM images of Pd nanoparticles with scale bars: (a) 20 nm, (b, c) 10 nm, (d, e) 5 nm, (f) 10 nm, (g, h) 20 nm, (i) 10 nm without AgNO 3; (j)–(l) 20 nm with the addition of 0.02 M AgNO 3. (m) Models of Pd nanoparticles give a good explanation of the experimental TEM images of the Pd nanoparticles.
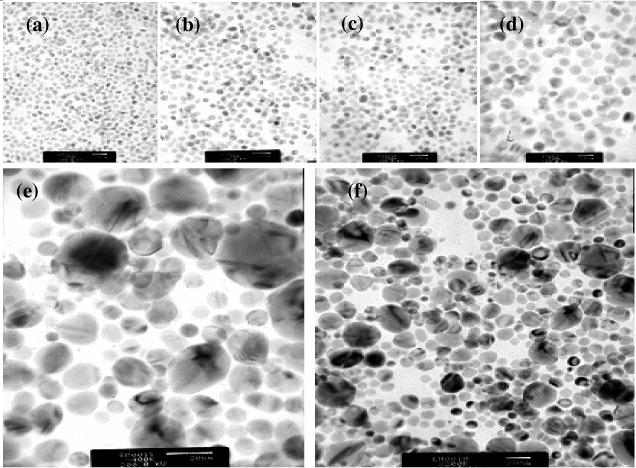
Pd nanoparticles of tetrahedral and octahedral shapes were found and they became larger in size. A lot of aggregations and clusterings of small nanocrystals possibly resulted in big porous Pd nanoparticles. Thus, EG plays the role of the structure-directing agent for the Pd nanoparticles. However, these Pd nanoparticles are still truncated at their corners and edges. The addition of a low concentration of 0.02 M AgNO 3 is very important for the appearance of the main shapes of the PVP–Pd nanoparticles and the controlled size less than 5–8 nm in figure 2(j). It was found that a silver cation has played the role of a structure-directing agent for controlling the formation of very stable polyhedral Pd nanoparticles from polyhedral seeds [11], and certainly this was also true for the case of the synthesis of Pd nanoparticles. The role of Ag + is to enhance the {100} growth, and/or to suppress the {111} growth, and Ag + is readily reduced by EG at high temperatures, resulting in reduced silver species, such as Ag 4 2+ clusters. Ag 4 2+ or Ag 0 can be adsorbed onto the surface of {100} facets and cations based on Pd nanoparticles and can easily be removed by organic solvents [11]. Besides the effect of AgNO 3, there are many other factors affecting the particle size [1], such as the PVP polymer, the pH, the reaction temperature, the EG and other experimental conditions. PVP has played an important role in controlling the nucleation and crystal growth rate of Pd nanoparticles. In particular, Pd nanorods were observed with the size ∼12 nm in figure 3(a) and the particle size of Pd nanoparticles was estimated as approximately ∼8–14 nm. However, with the addition of a large amount of 0.06 M AgNO 3, spherical and various other shapes are formed with the biggest size of about 40 nm in figures 3(e) and (f). They have some intermediate planes of spherical and quasi-spherical Pd nanoparticles that are twin and stacking fault nanostructures as Pd polycrystals. Moreover, the various twinned nanoparticles of decahedral and icosahedral shapes were observed and studied. Pd nanorods with the sizes of 47 nm and 16 nm were observed due to a high AgNO 3 content in figure 4(a). Irregular shapes of Pd nanoparticles appeared when we used a concentration of 0.02 M NaI in figure 4(b). Pd nanoparticles have been synthesized by the polyol method with the addition of 0.5 ml of 0.02 and 0.06 M FeCl 3.
Figure 3 TEM images of Pd nanoparticles with the addition of 0.02 M AgNO 3. TEM images of Pd nanoparticles with scale bars: (a) 20 nm (after 1 day), (b) 20 nm (1 week), (c) 20 nm and (d) 10 nm (2 weeks), respectively. (e, f) 20 nm with the addition of 0.06 M AgNO 3.
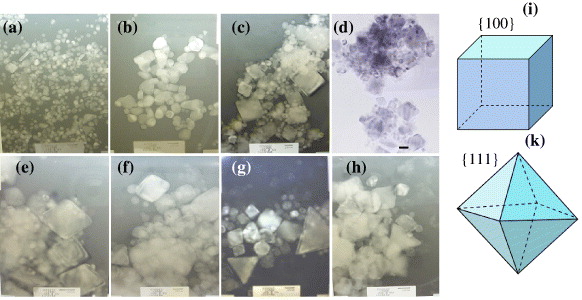
Figure 4 TEM images of large and various shapes of Pd nanoparticles with scale bars: (a) 20 nm (the addition of 0.5 ml of 0.06 M AgNO 3); (b) 20 nm (0.5 ml of 0.02 M NaI); (c, d) 20 nm (0.5 ml of 0.002 M FeCl 3); (e) 20 nm (0.5 ml of 0.02 M FeCl 3); (f–h) 20 nm (0.5 ml of 0.06 M FeCl 3). (i)–(k) cube and octahedron in TEM images of Pd nanoparticles in (c) and (e).
Beyond the existence of small spherical Pd nanoparticles, the tetrahedral and octahedral Pd nanoparticles become much larger in particle size. This led to Pd nanoparticles of various sizes and shapes being observed, such as a sharp tetrahedral shape (120 nm and 70 nm), a very sharp octahedral shape (60 nm and 47 nm) and other shapes in figures 3(c)–(f). The evidence of the formation of one very sharp corner of a tetrahedron or other shapes due to the aggregation and combination of smaller Pd nanoparticles (seeds) was found in figure 4(d). There are various trends in the formation of tetrahedrons and octahedrons of very sharp edges and corners from the agglomeration and combination of smaller nanoparticles. A higher concentration of AgNO 3, FeCl 3 and NaI was not good for controlling the size and shape of the Pd nanoparticles due to the aggregation problem of small Pd nanoparticles and the appearance of their irregular size and shape. Therefore, the amounts of AgNO 3, FeCl 3 and NaI used should be small. Clearly, the important thing was to avoid the formation of big clusterings of Pd nanoparticles during synthesis. We suggest that a small amount of AgNO 3 improves the growth mechanisms and formation of Pd nanoparticles and well-controlled size, as well as a good size distribution. In addition, the homogeneity of the solvent for preparing the solutions and controlling the size and morphology of the Pd nanoparticles is very important in our preparation procedure.
4. Conclusions
Pd nanoparticles were successfully synthesized by the reduction of Na 2 PdCl 4 with the use of EG as a reductant in the existence of PVP polymer. It was seen that the parameters of the supply rate of the precursor, temperature and time of synthesis reactions have been used to make Pd nanoparticles by the soft chemical method. In addition, the homogeneity of the solvent for the preparation of the stock solutions to synthesize and control the size and morphology of the Pd nanoparticles is very important in our preparation procedure. The addition of a small amount of AgNO 3 resulted in the improvement of the growth mechanisms and formation of good shapes of Pd nanoparticles.
Acknowledgments
We thank Yushi Tsutsui and Randy Jalem for their useful discussions and assistance in studying metal nanoparticles and their controlled synthesis at Nagoya Institute of Technology.