ABSTRACT
Understanding the evolution of organic molecules in ice grains in the interstellar medium (ISM) under cosmic rays, stellar radiation, and local electrons and ions is critical to our understanding of the connection between ISM and solar systems. Our study is aimed at reaching this goal of looking directly into radiation-induced processing in these ice grains. We developed a two-color laser-desorption laser-ionization time-of-flight mass spectroscopic method (2C-MALDI-TOF), similar to matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectroscopy. Results presented here with polycyclic aromatic hydrocarbon (PAH) probe molecules embedded in water-ice at 5 K show for the first time that hydrogenation and oxygenation are the primary chemical reactions that occur in astrophysical ice analogs when subjected to Lyα radiation. We found that hydrogenation can occur over several unsaturated bonds and the product distribution corresponds to their stabilities. Multiple hydrogenation efficiency is found to be higher at higher temperatures (100 K) compared to 5 K—close to the interstellar ice temperatures. Hydroxylation is shown to have similar efficiencies at 5 K or 100 K, indicating that addition of O atoms or OH radicals to pre-ionized PAHs is a barrierless process. These studies—the first glimpses into interstellar ice chemistry through analog studies—show that once accreted onto ice grains PAHs lose their PAH spectroscopic signatures through radiation chemistry, which could be one of the reason for the lack of PAH detection in interstellar ice grains, particularly the outer regions of cold, dense clouds or the upper molecular layers of protoplanetary disks.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
Astrophysical ices are water-rich amorphous ice grains with silicate core—a model put forward by Greenberg (1963) and corroborated by subsequent Infrared Space Observatory observations and recent Spitzer ice Legacy study (Gibb et al. 2000, 2004; Oberg et al. 2011). These water-rich ice grains of interstellar medium (ISM) are in amorphous form (Gibb et al. 2004), which is typically porous and could lead to some diffusion of even heavier atoms and molecules (di- and triatomic) into the pores. Thus, amorphous ice gives a unique opportunity to catalyze and participate in the chemical reactions involving accreted molecules, and reactive species produced within these amorphous ices (such as O, OH, and H) also contribute to overall chemistry during gas–grain collisions.
Our understanding of complex organic chemistry in interstellar ice grains (Boogert et al. 2011; Charnley & Rodgers 2008; Herbst & van Dishoeck 2009; Jones et al. 2011; Wakelam et al. 2010; Whittet et al. 2011) and protoplanetary disks (PPDs; van Dishoeck 2004; Vasyunin et al. 2008; Walsh et al. 2010) has improved tremendously in the past decades. The present understanding is that the universe is enriched with water-rich ices and hydrocarbon molecules in the form of aromatic structures as well as oxygen and nitrogen containing complex molecules (Greenberg & Caro 2000; Herbst & van Dishoeck 2009; Nuevo et al. 2010; Ruiterkamp et al. 2007; Schutte et al. 1993), some of which are building blocks of life (Bernstein et al. 2002; Caro et al. 2002; de Marcellus et al. 2011; Kuan et al. 2003). Polycyclic aromatic hydrocarbon (PAH) molecules such as naphthalene could be synthesized in cold interstellar environments through gas-phase collisional reactions (Carelli et al. 2011; Parker et al. 2012). Though several attempts were made to detect PAHs on cold interstellar ice grains, so far no positive detection has yet been made (Oberg et al. 2011), though ice, hydrogenated amorphous carbon (HAC), and PAH features have been observed (not necessarily coexisting in the same ice grain) in ultraluminous infrared galaxies (ULIRGs) with z ∼ 2 with the Spitzer Space Telescope (Sajina et al. 2009).
Severe impediments for both laboratory studies and observations in the infrared (IR) are the spectral features of organic molecules such as PAHs or simple methane, ethane, etc., being strongly overshadowed by the IR absorption bands of water-ice itself, making it difficult to track radiation-induced processing of organics in astrophysical ices. Recently, we have shown for the first time that the ultraviolet-visible (UV-VIS) spectral region is highly suitable for investigating strongly absorbing molecules like PAHs in ices (Gudipati 2004; Gudipati & Allamandola 2003, 2006). We have shown that water-ice facilitates ionization and lowers ionization energies of PAHs. More detailed kinetic data have also been subsequently retrieved from similar UV-VIS and IR experiments on ices containing PAHs (Bouwman et al. 2009, 2010, 2011a, 2011b). At the end of nearly a decade of optical spectroscopy of PAHs in astrophysical ice analogs, we can conclude that ionization of PAHs is evidently the first step in radiation processing. Subsequent chemistry results in hydrogenation and oxygenation reactions during warm-up studies (Gudipati 2004) or analysis of non-volatile residues at room temperature (Bernstein et al. 1999). However, detailed knowledge on the temperature these reactions get triggered is still missing. Do they occur already at 10 K? Or is there a temperature threshold at which reactions between PAH+ and H, O, or OH radicals occur in ice? These questions remain largely unanswered. Present understanding of how PAHs accreted onto ice grains in the ISM could evolve is still far from complete.
In order to circumvent these hurdles in the laboratory framework, without having to be constrained by spectroscopic band overlaps or weak absorptions or limited spectroscopic identification of product species, we built a matrix (water-ice) assisted two-color (IR and UV) laser-desorption, laser-ionization time-of-flight (2C-MALDI-TOF) mass spectrometer. The uniqueness of this instrument is that ices containing organics at any temperature between 5 and 200 K can be in-situ probed for their chemical content, enabling us to take "snap-shots" of the chemical evolution of organics in astrophysical ices without warming up or temperature-programmed desorption (TPD). At each temperature we now have the ability to determine relative abundances of reactive intermediates and products accurately. Desorption (ablation and desorption are used here to represent the same phenomenon of ejecting plumes of atoms, molecules, and clusters through excitation of O–H vibrational overtones of water at 2.94 μm wavelength) of water-ice containing organic or inorganic impurities—similar to interstellar ice grains—is achieved through an infrared laser. Desorbed plumes contain molecules and water clusters with trapped impurities, without damaging the chemical composition of the trapped species. In the plume, these species are instantaneously ionized using a UV laser and subsequently passed through a 100 cm long TOFMS to analyze the spectra of the constituent species. We have tested in the laboratory that the nanosecond-pulsed infrared or ultraviolet lasers that are used for ice ablation and ionization induce no additional reactions. The infrared laser puts most of its energy into water O–H vibrations, whereas the ultraviolet laser puts most of its energy into ionization and in molecular ions. Due to the high dilution (1:1000) of PAHs in H2O ice, PAHs and their radiation-processed species remain unaltered during IR laser ablation of the ice. We note here that laser ablation of water-ice containing organic and biomolecules has been reported in the literature either using one or two lasers (Baltz-Knorr et al. 2002; Belov et al. 1995; Berkenkamp et al. 1996; Hellwig et al. 2009; Mihesan et al. 2004). However, to the best of our knowledge, the studies reported here are the first to track radiation-induced chemistry of organic molecules in astrophysical ice analogs using laser desorption and laser ionization mass spectroscopy.
2. EXPERIMENTAL DETAILS
Recently we developed a novel laboratory 2C-MALDI-TOFMS instrument at the Ice Spectroscopy Lab (ISL) of the Jet Propulsion Laboratory that enabled for the first time to take a closer look into the chemical evolution of interstellar ice analogs containing organics (Figure 1). Ablation of water-rich cryogenic ices containing organics, metals, and minerals is achieved using a tunable infrared (IR) laser around 3000 nm (2.7–3.1 μm, 5 ns and ∼8 mJ pulse−1, OPOTEK, Vibrant IR) wavelength. The ice and water cluster plumes that are formed from IR laser ablation carry with them the embedded atoms, radicals, and molecules (big or small) into the gas phase without damaging the species embedded. A second UV laser at 266 nm (4th harmonic of Nd-YAG laser, 5 ns, and ∼3 mJ pulse−1, Quantel Brio) focused on the plume through a 150 mm plano-convex lens is then fired with a time delay of 4–10 μs to intercept this plume and ionize atoms and molecules in that plume through resonance-enhanced multiphoton ionization (REMPI). Once ionized, these species are then accelerated into and drifted through the TOF tube for mass-separation and detected using a micro channel plate (MCP) detector (from Jordon TOF systems). This process is depicted schematically in Figure 1. Cryogenic ices are prepared through gas-phase co-deposition of premixed PAH with water (approximately 1:1000). Once films of ∼1 μm thick are prepared, these ices are then analyzed using the 2C-MALDI-TOFMS method described above, subjected to Lyα at 121.6 nm and H2 continuum (160 nm; hydrogen microwave discharge lamp; Opthos Instruments; flux: ∼1015 photons s−1 cm−2) by rotating the probe in the vacuum chamber.
Figure 1. Schematic representation of MALDI-TOFMS instrument developed at the JPL's Ice Spectroscopy Laboratory (ISL). The substrate is rotated for vacuum ultraviolet (VUV; predominantly Hydrogen Lyα at 121.6 nm) irradiation and subsequent 2C-MALDI-TOFMS measurements in the vacuum chamber. An infrared laser desorbs ice and its components to plume without damaging the embedded organics. An ultraviolet laser ionizes these organics, and the ions are accelerated through the time-of-flight tube to a micro channel plate (MCP) detector. Download figure:
3. RESULTS AND DISCUSSION
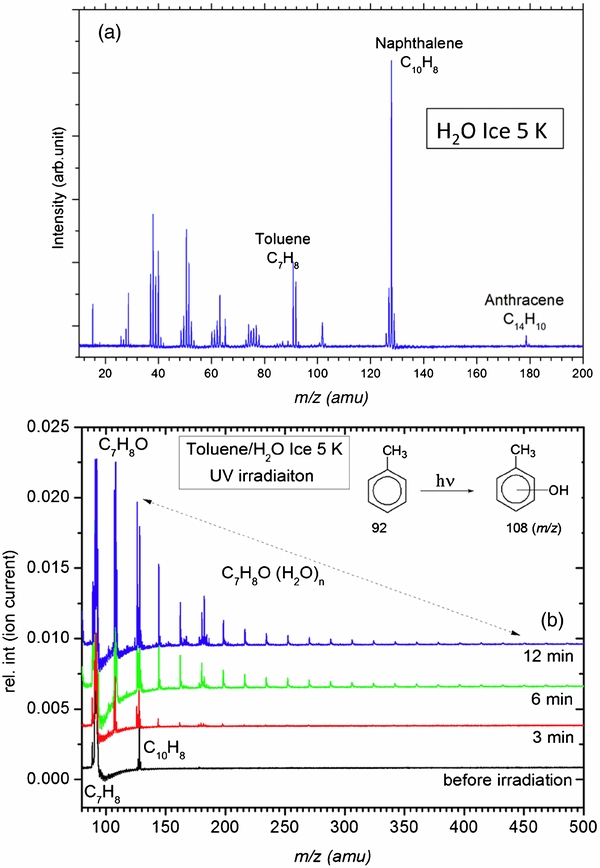
Neutral PAHs in ice spectra. An example of the 2C-MALDI-TOFMS spectrum of mixed toluene (C7H8), naphthalene (C10H8), and anthracene (C14H10), co-deposited in amorphous ice at 5 K is shown in Figure 2(a). Molecular ion peaks of toluene (92), naphthalene (128), and anthracene (178) are clearly seen, in addition to their fragmentation pattern. For example, the fragmentation pattern of toluene and naphthalene is identical to the one that can be found in the literature (http://webbook.nist.gov/chemistry/). We noticed that the fragment mass intensity increases with increasing UV laser power due to excess of energy deposited into the ionized molecule that leads to fragmentation of molecular ion during ionization event. We have not observed chemical modifications of neutral PAHs when kept in the dark subsequent to deposition (as seen in Figure 2(a)) even at very strong IR laser ablation and UV laser ionization conditions—proving that the 2C-MALDI-TOFMS technique does not chemically alter the probe PAHs during the ablation and ionization processes.
Figure 2. (a) 2C-MALDI-TOFMS spectrum of toluene, naphthalene, and traces of anthracene co-deposited with H2O to form astrophysical ice analog at 5 K. Fragmentation mass peaks are relatively strong due to higher 266 nm ionization laser flux. (b) MALDI-TOFMS spectra of toluene embedded in water-ice at 5 K before and after radiation processing with Lyα photons (hydrogen lamp). Hydroxytoluene (m/z = 108 amu) remains in water clusters that are desorbed by the infrared laser, even after ionization with the UV laser due to strong hydrogen bonding. Toluene (non-polar molecule) alone does not bind with water, as seen from the lack of cluster formation before UV-irradiation. Download figure:
Hydroxylation of PAHs at 5 K under UV radiation. Soon after the ices were subjected to UV light (hydrogen flow-discharge lamp), we noticed additional mass peaks that correspond to hydrogenation and hydroxylation (insertion of O atom into the C–H bond to form C–OH functionality and structural isomers thereof) of these aromatic molecules. Addition of mass 16 (oxygen) results in the formation of hydroxylated (OH) toluene, known as cresol (Figure 2(b)). Addition of O to the CH3 group of toluene results in benzylalcohol, which can only be a minor product based on the intensity of the fragmentation at 77 and 79 amu, which should be the strongest peak for benzylalcohol. In all our spectra the intensity of 79 amu was significantly less than the 108 amu peak that corresponds to hydroxylated toluene. Hence we conclude that the addition of the O atom to the aromatic ring is more probable than the aliphatic group. Once hydroxylated, these aromatic molecules form strong hydrogen bonding with the surrounding water. As a result, hydroxylated toluene (cresol) embedded in ice nanoparticles (clusters of H2O molecules up to 55 water molecules are observed in the 2C-MALDI-TOFMS under our present low-power conditions). These results show that once ionized and hydroxylated PAHs become more strongly bound to ice than before.
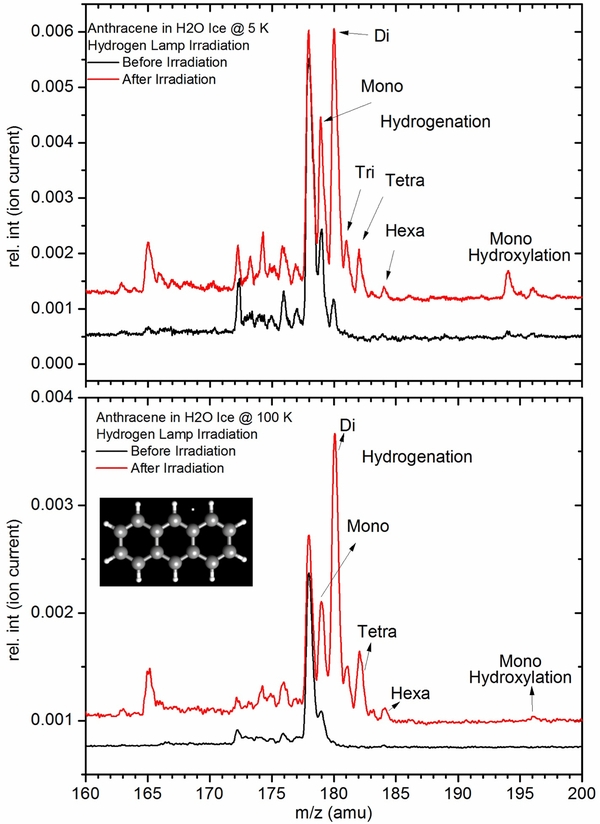
Hydrogenation of PAHs at 5 K under UV-radiation. Surprisingly, all the PAHs studied are readily hydrogenated during UV-irradiation at 5 K. A careful analysis revealed that hydrogenation efficiency increases at higher temperature (Figure 3). Anthracene can be hydrogenated with up to 10 hydrogen atoms (see Figure 3 for structure) without losing the ring structure. We observed up to six hydrogen atoms added to anthracene, equivalent to hydrogenation of two aromatic rings leaving the benzene-like aromatic ring unhydrogenated. The addition of two hydrogen atoms at a time has higher intensity, because each double bound can take two hydrogen atoms, leaving the conjugation of the rest of the molecule intact, whereas an odd number of hydrogen atom addition results in less stable radicals. Generally, hydroxylation has shown to be less temperature sensitive than hydrogenation. However, the very fact "that O atom insertion occurs at 5 K into PAH molecules surrounded by water-ice" is counterintuitive, but shows that aromatic hydrocarbons (and perhaps non-aromatic as well) can undergo chemical modifications in ice surroundings under UV-radiation.
Figure 3. Hydrogenation of anthracene at 5 K (top) and at 100 K (bottom). Addition of a set of two hydrogen atoms (even number) is more favored than an odd number of hydrogen atoms due to the aromatic nature of the molecule. Hydrogenation efficiency is higher at 100 K than at 5 K. Hydroxylation though seems to be more efficient at 5 K than at 100 K is generally observed to be temperature independent. Download figure:
Proof that IR and UV lasers of MALDI do not induce chemical processes. Initially we were skeptical about the damage or chemical modifications that the IR laser could cause when focused on the ice for ablation. For this reason, we carried out a series of tests, experiments, and analysis as summarized here. IR desorption wavelength at 2.94 μm selectively excites O–H vibrations of H2O-ice, generating plumes of water molecules, nanoclusters (several H2O molecules), and larger ice particles. Before UV-irradiation, PAH molecules are intact in the 2C-MALDI-TOFMS, indicating that no chemistry occurs in the plumes involving neutral PAHs and H2O molecules (Figure 2). However, after UV-irradiation that generates ionized PAH radical cations and radicals such as H, O, and OH, collisional chemistry in the plume could not be ruled out. There are reports in the literature that collisions in the plumes could lead to secondary reactions (Johnson & LeBeyec 1998; Knochenmuss & Zenobi 2003). Plume particles are expected to travel at hundreds to thousands m s−1 (Liang et al. 2011) velocity and most of the plume expansion occurs within a few hundred nanoseconds during which time collisions could occur. However, well-established reports on similar "laser desorption of ice" methods such as matrix assisted pulsed laser evaporation (MAPLE) clearly demonstrated that even at much higher laser fluxes (compared to the IR laser flux used in this experiment), organic molecules embedded in water-ice are not damaged during pulsed-laser evaporation/desorption of ice (Pique 2011; Toftmann et al. 2005).
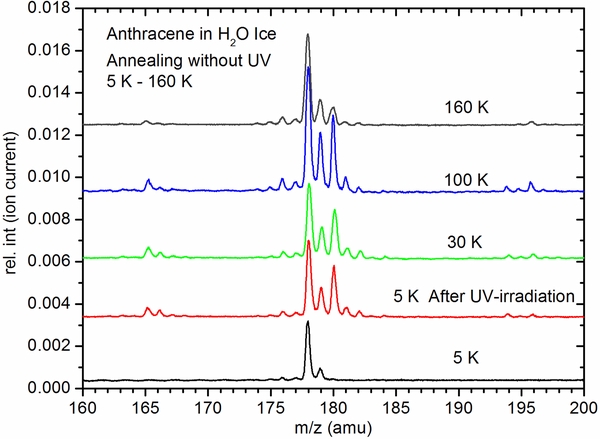
In our experiments, cryogenic water-ices are probed. IR laser fluencies are kept low (∼5 mJ pulse−1) and focused on ∼0.2 mm diameter on the ice. The fragmentation pattern of neutral PAHs during these studies are identical to gas-phase mass spectra from the NIST's database. Based on these facts and the data presented here so far, it is clear that (1) neutral PAHs do not undergo reactions during the IR laser ablation, (2) with increasing UV radiation processing, increasing hydrogenation and hydroxylation are seen in the MALDI-TOFMS spectra, and (3) termination of UV radiation processing does not alter the intensities of reaction products even at increasing temperatures. At ∼10 K interstellar ice temperatures, H atoms can be trapped in amorphous ice (Matar et al. 2008; Watanabe et al. 2010). However, H atoms that are generated during the UV photolysis of water-ice do not survive at 100 K—they react immediately with the surrounding atoms and radicals. Hence, contribution of reactions involving H atoms and PAH radical cations after IR laser desorption in the ice plume, if any, should only be a minor part of the observed hydrogenation reactions at 100 K. At 5 K, though such plume chemistry cannot be ruled out completely, experimental results show that higher IR laser fluence does not cause the chemistry—independent of the plume characteristics. If trapped radicals and PAH ions were to react in the plume, increase in temperature should result in changes in the mass spectra due to changes in concentrations of H, O, and OH radicals in the ice at different temperatures. We carried out experiments in which after UV irradiation at 5 K, ices were warmed up and 2C-MALDI-TOFMS spectra were collected at different temperatures (Figure 4). Lack of further change in product distribution during warm-up after UV irradiation of PAHs in ice at 5 K (Figure 4) clearly demonstrates that spectra measured at 5 K are due to products in the ice before IR laser desorption than due to plume chemistry. Future experiments are planned to look more closely into these processes.
Figure 4. UV-induced hydrogenation of anthracene at 5 K and subsequent warm-up to 160 K. Except at 160 K where most of the hydrogenated anthracene is depleted due to the onset of ice sublimation, no change in the relative intensities of anthracene (left most peak at 178 amu) and its hydrogenated products is observed up to 100 K, clearly demonstrating that IR and UV lasers used in the 2C-MALDI-TOFMS instrumentation do not cause plume chemistry. Download figure:
4. ASTROPHYSICAL IMPLICATIONS
One of the key results of our study is that multiple hydrogenations of PAHs occur even at as low as 5 K in water-rich ices. This observation is in agreement with gas phase (Snow et al. 1998) and theoretical studies (Bauschlicher 1998; Hirama et al. 2004). Unlike neutral PAH molecules, ionized PAH radical cations react exothermically with H atoms even at 5 K. UV radiation processing of water-rich ice produces significant amount of H, O, and OH radicals, which could immediately react with ionized PAHs in the vicinity even at very low interstellar temperatures. Consequently, though PAHs are expected to coexist with interstellar ices, they could be processed to hydrogenated and oxygenated hydrocarbons, losing quickly their chemical identity when adsorbed on interstellar ice grains. With addition of two hydrogen atoms, a naphthalene molecule loses its characteristic PAH properties in the UV-VIS spectral region and results in mixed aromatic (benzene ring) and aliphatic (hydrogenated ring) properties in the IR region. As shown in our studies, multiple hydrogenations of PAHs accreted on ices can occur under UV radiation, which can be accelerated in environments that are enriched with H atoms that accrete on to the surface of these ice grains. This chemical vulnerability of ionized PAHs to undergo exothermic or barrierless modifications with H, O, and OH radicals, could be the cause behind the lack of observation of PAH signatures in interstellar ices (Oberg, et al. 2011). The lifetime of PAHs, once they reach ice grains, will be limited—based on the radiation, photon, H atoms, and ions.
Dense molecular clouds (DMCs) are expected to have the least ionizing UV photon flux, which is mainly due to cosmic-ray photoionization of gas-phase hydrogen, resulting in hydrogen atom and molecular emission. This local ionizing UV radiation in DMCs is estimated to be ∼1350 photons cm−2 s−1 (Prasad & Tarafdar 1983), whereas protoplanetary or circumstellar environments receive much higher UV flux of several orders of magnitude higher than the DMC PT (Prasad–Tarafdar) flux (Throop 2011). Under our experimental conditions, with ∼1015 photons cm−2 s−1, in about 10–15 minutes (∼1018 photons cm−2) of irradiation, >50% of PAHs are multiply hydrogenated in a film of ∼1 μm thickness (close to the dimensions of interstellar ice grains). In DMCs, with no external radiation present, this would mean ∼1015 s or ∼3 × 107 yr for depletion of PAHs. While the mean lifetimes of DMCs are ∼109 yr, once the molecular gas freezes out, the lifetimes are only ∼106 yr. Thus, the outer regions of cold, dense clouds or the upper molecular layers of PPDs are regions in which this process could be effective, whereas in DMCs PT-flux alone may not be sufficient to completely hydrogenate PAHs and accretion and penetration of H atoms into ice grains may be critical. Radiation-induced hydrogenation and oxygenation processes described here could deplete PAH population in ices in DMCs to a lesser extent. In interstellar ices and circumstellar ices of protostars and more evolved stars with much higher UV photon fluxes, the lifetime of PAHs would be several orders of magnitude shorter than ∼107 yr. PAHs on the surfaces of icy bodies from Kuiper Belt objects (KBOs) to Jupiter family comets (∼50–5 AU) would be radiation processed even faster. The only way PAHs survive on these bodies would be at depths beyond the reach of surrounding electron or photon radiation. PAHs found in comet outgassing must be coming from the subsurface. The chances for PAHs to survive would be higher in the environments that are devoid of water-ice or hydrogen—such as asteroids, meteoroids, and interstellar dust particles (IDPs). Observation of a variety of PAHs on these objects (Botta et al. 2008; Clemett et al. 1993) confirms our thesis.
6. CONCLUSIONS
First in-situ insight into radiation-induced chemical modification of PAHs in astrophysical ice analogs beyond ionization process has been successfully derived through novel 2C-MALDI-TOFMS studies, whereby UV-radiation-processed PAHs in ice were desorbed by an IR laser and ionized by a UV laser. These studies show that even at DMC temperatures of ∼10 K, local UV photons can induce hydrogenation and oxygenation reactions in the ice grains. It is proposed that failure to detect PAHs in high-UV flux regions such as the outer regions of cold, dense clouds or the upper molecular layers of PPDs could be due to rapid radiation processing of these molecules into non-aromatic hydroxylated and hydrogenated hydrocarbons in ices. The studies presented here also show that interstellar ice grains at low-temperatures are nuclei of chemical transformation of organic molecules even at very low temperatures. At higher temperatures corresponding to KBOs and Jupiter family comets, PAHs would be oxidized and hydrogenated much more rapidly, leaving little chance to detect them on icy surfaces.
This research was enabled through partial funding from JPL's DRDF and R&TD funding for infrastructure of the "Ice Spectroscopy Laboratory" at JPL, NASA Spitzer Science Center, NASA funding through Rosetta US Science Team, and NASA Astrobiology Institute Node ''Icy Worlds''. This research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration.