ABSTRACT
Surface reactions between carbon monoxide and non-energetic hydroxyl radicals were carried out at 10 K and 20 K in order to investigate possible reaction pathways to yield carbon dioxide in dense molecular clouds. Hydroxyl radicals, produced by dissociating water molecules in microwave-induced plasma, were cooled down to 100 K prior to the introduction of CO. The abundances of species were monitored in situ using a Fourier transform infrared spectrometer. Formation of CO2 was clearly observed, even at 10 K, suggesting that reactions of CO with OH proceed with little or no activation barrier. The present results indicate that CO2 formation, due to reactions between CO and OH, occurs in tandem with H2O formation, and this may lead to the formation of CO2 ice in polar environments, as typically observed in molecular clouds.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
Since the first detection of solid CO2 in the interstellar medium (ISM; d'Hendecourt & de Muizon 1989), extensive astronomical observations have been carried out. These works have reported a high abundance of CO2 ice embedded in icy mantles from a number of sources (Gerakines et al. 1999; Gibb et al. 2000). Recent observations obtained with the Spitzer Space Telescope have provided information suggesting that the CO2 column density is high (<15%; Pontoppidan et al. 2008) and almost constant relative to H2O (Whittet et al. 2007; Pontoppidan et al. 2008). Because the observed solid CO2 abundance cannot be explained only by gas-phase reactions (Hasegawa et al. 1992) and because a large amount of solid CO is observed in interstellar ice mantles (Gibb et al. 2004), it is widely accepted that CO2 in ice mantles is formed by surface reactions. Representative pathways to the formation of CO2 involve two CO molecules (reaction (1)), CO molecules with atomic oxygen (reaction (2)), and CO molecules with hydroxyl radicals (reaction (3)):
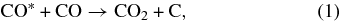

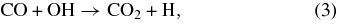
where CO* represents an internally excited CO molecule.
Reaction (1) has been experimentally demonstrated to occur by photolysis with Lyα photons, irradiation with 200 keV protons (Loeffler et al. 2005), and 5 keV electron bombardment (Jamieson et al. 2006; Bennett et al. 2009). Reaction (2) has also been studied; however, the formation of CO2 is confirmed only by temperature-programmed desorption experiments using thermal (Roser et al. 2001) and energetic (Madzunkov et al. 2006) O atoms. Reaction (3) has been theoretically (e.g., Yu et al. 2001; Senosiain et al. 2003; Varelo et al. 2004; Song et al. 2006) and experimentally (e.g., Frost et al. 1993; Laster et al. 2000) investigated in gas phase. The potential surface for the gas-phase reaction (3) contains two barriers: one in the entrance channel (OH + CO → HOCO) and another in the exit channel (HOCO → CO2 + H). However, the existence and the height of the energy barriers remain under debate. Watanabe & Kouchi (2002) investigated reaction (3) by inducing ultraviolet irradiation to H2O/CO binary ice, where OH radicals were produced by photodissociation of H2O. In general, photons at around 130–120 nm induce photodissociation of H2O ice with an excess energy of ∼5 eV, which is partitioned into the translational and rovibrational energies of OH. Such energetic OH radicals can overcome the activation barrier for reaction (3), which is estimated to be approximately 500 K (Yu et al. 2001; value varies in the literature).
Recent detections of abundant CO2 in dense clouds observed toward background stars, in which the UV field is relatively weak, suggest that some routes of formation without UV irradiation may also contribute to the CO2 formation in these environments (Bergin et al. 2005; Knez et al. 2005; Whittet et al. 2007), although the cosmic-ray-induced photons may still be effective for triggering chemical reactions inside dark clouds (Garrod et al. 2007). Reaction (3) is a possible route to the formation of CO2 in dense clouds, because the barrier height of reaction (3) is not high (Yu et al. 2001), and it is expected to lower when H2O is present (Y. Osamura 2008, private communication). Herein, we present the results of CO2 formation by surface reactions of CO molecules with non-energetic OH radicals produced by dissociating H2O molecules.
2. EXPERIMENTAL DETAILS
Experiments were performed using the Apparatus for SUrface Reaction in Astrophysics (ASURA) system. ASURA consists of a main chamber, an atomic source, and a Fourier transform infrared spectrometer. Details of this apparatus have been described previously (Watanabe et al. 2006; Nagaoka et al. 2007). At the center of the main chamber, a mirror-finished aluminum (Al) substrate is mounted on the cold head of an He refrigerator. The base pressure of the main chamber is ∼10−10 torr, but it reaches (1–2) × 10−7 torr during operation of the atomic source. Hydroxyl radicals (OH), together with atomic hydrogen (H) and oxygen (O), were dissociated from H2O molecules by microwave-induced plasma (Timmermans et al. 1998) in a Pyrex tube. The H2O dissociation may also lead to direct formation of H2 and O (Hama et al. 2009). The H2O fragments (H, O, OH, and H2) were transferred via a series of poly(tetrafluoroethylene) and aluminum tubes to the substrate, and then cooled to 100 K in an aluminum tube that was connected to another He refrigerator. The deposition rate of OH radicals cannot be directly measured in the present experiment; however, the upper limit of the deposition rate can roughly be estimated to be 1.5 × 1013 radicals cm−2 s−1, which is equal to the H2O deposition rate when the microwave source is turned off. CO molecules were introduced into the main chamber through a capillary plate located approximately 5 cm from the substrate, with an incident angle of 30° to the normal of the substrate surface. The deposition rate of CO molecules is 1.4 × 1013 molecules cm−2 s−1. The deposition rate was obtained by the initial slope of the increase in column density with time, when each molecule was deposited on the surface at 10 K. The statistical error of the deposition rate is less than 5%. The reaction products were monitored in situ by infrared reflection–absorption spectroscopy with a resolution of 4 cm−1 through the spectral range between 700 cm−1 and 4000 cm−1. Species desorbing from the substrate were monitored using a quadrupole mass spectrometer (QMS). The temperature of the Al substrate was maintained at 10 K or 20 K during each experiment. Each experiment was performed for 120 minutes.
3. RESULTS AND DISCUSSION
Under conditions in which CO molecules are absent, the H2O fragments react only with each other, resulting in the formation of O3, H2O2, and H2O, as observed in the IR spectrum with the typical absorptions at 1039 cm−1, 1395 cm−1, and 1648 cm−1, respectively. Detailed assignments of these molecules have been reported elsewhere (Lannon et al. 1971; Gerakines et al. 1995; Bennett & Kaiser 2005). The yield of H2O2 and O3 varies with substrate temperature; therefore, it is expected that these molecules are formed on the surface, not in the gas phase. The QMS revealed that H2 and O2 are contained in gases from the source. Trace amounts of CO2, which may have already been absorbed on the inner wall of the main chamber, were detected in the IR spectrum even before any reactive partners were admitted in the chamber (hereafter, this is referred to as the blank). Assuming that H2O2 is formed only by OH + OH → H2O2 in the blank experiment, the lower limit of the OH deposition rate was roughly estimated to be 8.5 × 1012 radicals cm−2 s−1. The ratio of the OH and CO deposition rates (OH/CO) was about 0.8, where the OH deposition rate is a value intermediate between upper and lower limits.
When CO molecules are simultaneously introduced together with the H2O fragments onto the substrate, the peak at 2344 cm−1 corresponding to the C = O stretching mode of CO2 molecules (Gerakines et al. 1995) increases in intensity (Figure 1(a)). The formation of CO2 is expected to occur via either reaction (2) or (3), or both, whereas reaction (1) can be excluded because CO* is absent under the present experimental conditions. In order to more clearly elucidate the reaction pathways, the following experiment was conducted: atomic O produced from O2 molecules in microwave-induced plasma was codeposited with CO molecules on the Al substrate at the same temperature (10 K or 20 K). The formation of CO2 was not observed under these conditions. The only product observed consisted of O3 molecules, which are formed by the reaction of O2 molecules with atomic O present on the substrate. This result indicates that reaction (2) does not occur in this temperature range. Roser et al. (2001) performed a similar experiment and observed CO2 formation by QMS only when CO/O mixed ice was covered with 100 monolayers (1 monolayer = 1015 molecules cm−2) of H2O ice, followed by increasing the substrate temperature above 150 K that forced CO and O to migrate through the ice layer and encounter on adsorption sites in water pores. Therefore, it is expected that reaction (2) can only proceed with the presence of water ice acting as a catalyst.
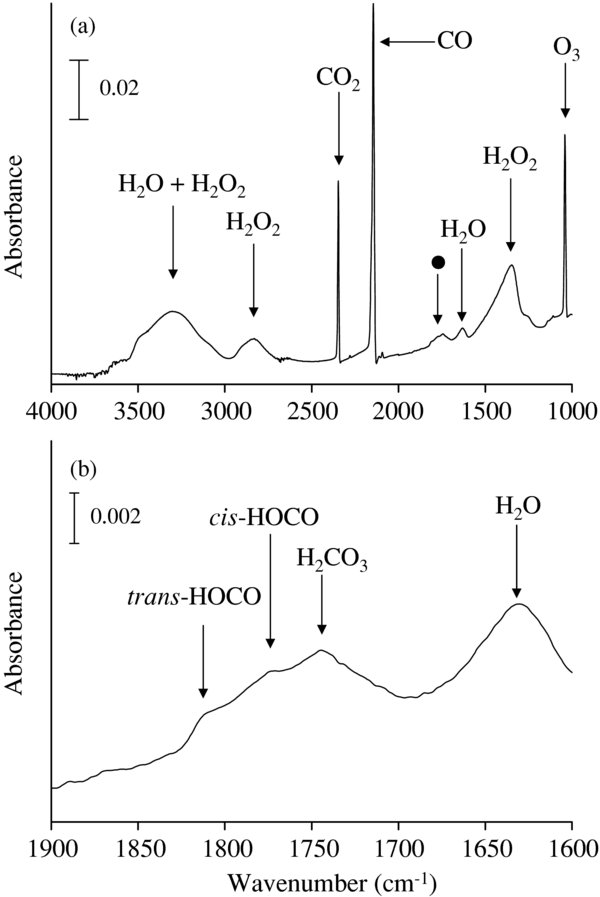
Figure 1. IR spectra of products for OH/CO of 0.8 at 10 K after 120 minutes: (a) 4000–1000 cm−1 and (b) 1600–1900 cm−1. Filled circle in panel (a) represents absorption by carbon-bearing species which are further described in panel (b).
Download figure:
Standard image High-resolution imageIn addition to CO2, a new peak was observed at 1745 cm−1 (Figure 1). There are several possible species that have absorptions in this region (for example, H2CO and HCOOH). Although our QMS data imply both H2CO and HCOOH could be present in products, the peak was attributed to carbonic acid (H2CO3) for the following reasons: the peak remained present even after heating up to 220 K, the shape and position of the peak resemble those of H2CO3 (Gerakines et al. 2000; Zheng & Kaiser 2007), and the signal for H2CO3 (m/z = 62) was observed in the QMS data when the substrate is heated to higher than 220 K. Experiments to elucidate the formation of H2CO3 are currently underway and detailed discussion on the formation of H2CO3 will be described in a future publication. HCO, H2CO, and CH3OH were not positively identified in the IR spectra under all conditions. This indicates that hydrogenation of CO did not occur under the conditions, suggesting that very little atomic H is contained in the H2O fragments and/or most of atomic H reacted to form H2 on the surface. Furthermore, when O2 molecules were introduced together with the H2O fragments onto the substrate, formation of H2O and H2O2 was negligible under that condition. If atomic H exists in the H2O fragments, O2 should react with the atomic H to yield H2O2 and H2O (Miyauchi et al. 2008; Ioppolo et al. 2008). These results support the above suggestion.
The IR spectrum shows two small peaks at 1774 cm−1 and 1812 cm−1 (Figure 1(b)). Similar peaks have been observed at 1797 cm−1 and 1833 cm−1 during vacuum-ultraviolet photolysis of H2O in a CO matrix at 14 K (Milligan & Jacox 1971). Although the peak positions differ slightly from the literature (about 20 cm−1), the interval between these two peaks is quite similar (∼ 36 cm−1). Milligan & Jacox (1971) proposed that these peaks are derived from cis- and trans-HOCO radicals produced by the reaction of OH with the CO matrix, respectively. The existence of the intermediate species has also been proposed by a series of theoretical studies (e.g., Yu et al. 2001; Senosiain et al. 2003; Varelo et al. 2004; Song et al. 2006). These two peaks were found to disappear when the sample was warmed up to >40 K, which is consistent with observations by Milligan & Jacox (1971). Therefore, these peaks may be attributed to cis- and trans- HOCO radicals, respectively.
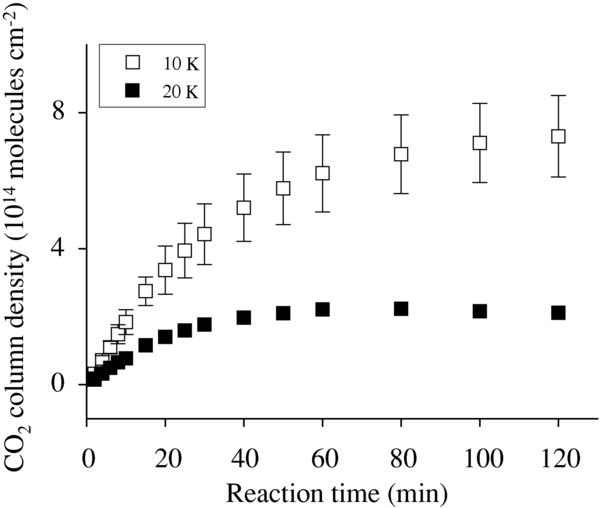
The column density of CO2 formed was calculated from peak areas and the previously reported integrated band strength, as described in Hidaka et al. (2007). A correction for the light path with IR incident angle of about 83° was made for this calculation. The band strength used was 7.6 × 10−17 cm molecules−1, which was obtained from transmission absorption spectroscopy of pure CO2 ice (Gerakines et al. 1995). It is well known that the band strength varies with the temperature and the ice composition. Therefore, the calculated CO2 column density may suffer from some errors because the reaction contains a number of different products, including H2O2, H2O, and O3 for the present experiment. In addition, our estimates of column density using reflection–absorption spectroscopy may contain some errors due to the use of band strength obtained by transmission spectroscopy (Oba et al. 2009). Nevertheless, it is expected that the difference in the CO2 band strength and the different IR methods are not so critical as to influence our discussion in the present study. Figure 2 shows plots of CO2 column densities with reaction time, in which CO2 detected in the blank has been subtracted. The spectrum shows that CO2 column densities reach saturation with time. This observation could be explained as follows. Since CO and OH are considered to minimally migrate at <20 K, reaction (3) may occur only when both species are adsorbed very close to each other on the surface. As the reaction proceeds, significant amounts of H2O2, H2O, and O3 are produced, which may lead to the formation of an icy layer having a surface area much larger than the original substrate. For example, at the initial 10 minutes of codeposition, the Al substrate is covered with approximately 20 ML of ice consisting of reaction products and CO. Therefore, the number of surface sites where CO and OH can be adsorbed increases, resulting in a decrease in the frequency of encounters between CO and OH. In fact, the CO2 column density linearly increased with time when reaction (3) was demonstrated at 40 K, where CO and OH should have higher mobility than 10 K and 20 K. This result supports our explanation for the CO2 profiles at lower temperatures.
Figure 2. Variations in CO2 column densities with time for OH/CO of 0.8 at 10 K and 20 K. Symbols include statistical error.
Download figure:
Standard image High-resolution imageThe conversion factor R of CO into CO2 through reaction (3) is defined as
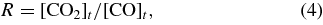
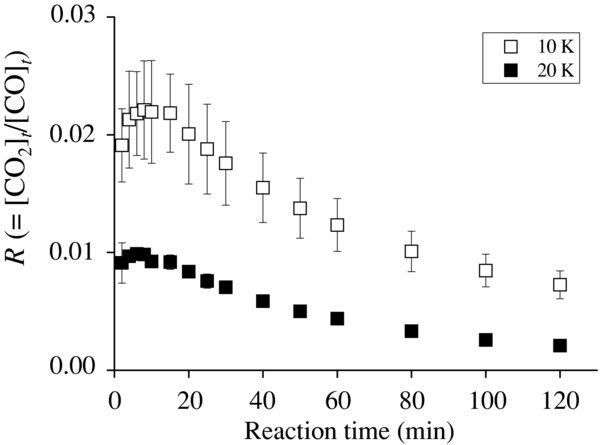
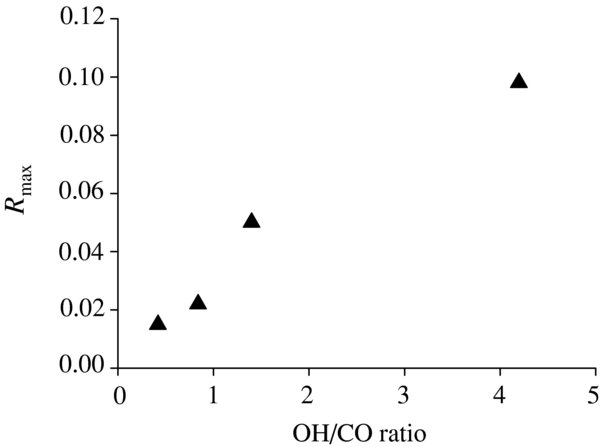
where [CO2]t and [CO]t represent the amount of the CO2 product and deposited CO (in cm−2) at time t, respectively. Figure 3 shows variations in the value of R with time. The maximum values of R (Rmax) are 0.02 and 0.01 at 10 K and 20 K, respectively. The values of R tend to decrease with time after ∼10 minutes (Figure 3) because the column density of CO2 reaches saturation (Figure 2) as described above, while CO is continuously introduced onto the substrate. In order to investigate the dependence of OH/CO ratio on the Rmax, the experiments were preformed with the different OH/CO obtained by changing the CO deposition rate. The value of Rmax increases with increasing OH/CO, which ranges from 0.02 (OH/CO = 0.4) to 0.10 (4.2) at 10 K (Figure 4). The Rmax values here obtained are comparable to the percentage of CO2 formed from CO when interstellar ice analogs containing CO are subjected to an energetic ion irradiation (7.2%–56.7%; Ioppolo et al. 2009) and when solid CO is irradiated by photons or protons (2.0%–6.4%; Loeffler et al. 2005). Therefore, it is expected that reactions of CO with non-energetic OH radicals are important routes to the formation of CO2, even when compared with energetic processes.
Figure 3. Variations in values of R, defined as [CO2]t/[CO]t, with a time for OH/CO of 0.8. Symbols include statistical error.
Download figure:
Standard image High-resolution imageFigure 4. Variations in values of Rmax with OH/CO at 10 K, where the OH deposition rate is a value intermediate between upper and lower limits.
Download figure:
Standard image High-resolution imageHydroxyl radicals (OH) obtained by dissociating H2O are thought to be rovibrationally excited. It is known that OH radicals in the excited vibrational level ν = 1 have energy of about 5100 K (Dodd et al. 1990). Reactions with energetic OH in the absence of dissociating UV photons in dense clouds would not be a great astrophysical importance, but if a reaction occurs when OH is energetic, little temperature dependence is expected for CO2 formation (Watanabe et al. 2007). However, profiles of the CO2 column density vary significantly with temperature (Figure 2). In addition, formation rates and yields of H2O2 and O3 differ by a factor of 2 at different temperatures. These features clearly indicate the temperature dependence of the OH-related reactions. Therefore, OH radicals used in our experiments are thought to be in the ground state mainly because the initial excess energy of the reacting partners is thought to be released through their collisional interaction with the cold aluminum pipe (100 K) used to transfer them onto the substrate. Any residual excess energy OH radicals may still have when they reach the surface should be thermally relaxed on the cold (10 or 20 K) surface during the accommodation process before reacting with CO molecules.
Goumans et al. (2008) proposed that reaction (3) does not yield CO2 when the excess energy of the intermediate HOCO radicals formed in reaction (3) is partially dissipated to the surface. Instead, they proposed that the HOCO can subsequently react in a barrierless manner with an additional atomic H to yield CO2 + H2, H2O + CO, or HCOOH with the same statistical branching ratio. If this were the case, reaction (3) should give a 1:1 ratio between HCOOH and CO2. However, the present results show that formation of HCOOH is negligible, and that CO2 is actually formed in reaction (3). Therefore, HOCO radicals formed are thought to further dissociate into CO2 + H without additional atomic H or it might be that the production of CO2 + H2 is dominant over the other two possible channels proposed by Goumans et al. (2008). The first alternative suggests that the HOCO radicals have enough excess energy to overcome the exit channel in reaction (3) and/or the reaction proceeds through quantum tunneling (Frost et al. 1993; Zhu et al. 2001).
4. ASTROPHYSICAL IMPLICATIONS
Astronomical observations toward young massive stars with Infrared Space Observatory (ISO) have shown that a large amount of solid CO2 exists in a polar (H2O-rich) environment (Gerakines et al. 1999). Such conditions are favorable for CO2 formation by reaction (3) because reactions of OH radicals are also related to H2O formation:

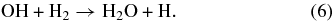
Therefore, CO2 formation through reaction (3) can occur in tandem with reactions (5) and (6). Cuppen & Herbst (2007) proposed that in dense clouds, reaction (6) is a common route to the formation of H2O. Formation of CO2 through reaction (3) should be favored over reaction (6), because formation through reaction (6) should proceed at a lower rate than reaction (3) due to the relatively high activation barrier (∼2100 K, Atkinson et al. 2004), which requires a quantum tunneling process at very low temperatures. On the other hand, reaction (3) has little or no activation barrier, as described earlier. Furthermore, our present results suggest that CO2 formation does not necessarily require the existence of H2O on the grains unlike UV-induced CO2 formation in an H2O/CO binary ice (Watanabe & Kouchi 2002). Therefore, CO2 formation (reaction (3)) may proceed in tandem with H2O formation (reactions (5) and/or (6)) at the early stage of mantle evolution in dense clouds. This would subsequently lead to the formation of CO2 ice in a polar environment, as proposed in previous studies (Bergin et al. 2005; Goumans et al. 2008).
In the present experiment, the Rmax value was found to increase with increasing OH/CO (Figure 4). If the OH/CO in the ISM is evident, we can roughly estimate the efficiency of reaction (3) in the ISM. However, it is not easy to estimate the actual value of OH/CO in the ISM. Assuming that one H2O molecule is produced from one OH radical (reactions (5) and (6)) on the grain surface and that CO is supplied only from the gas phase, the ratio of H2O and CO abundances in interstellar ices may be closely related to the OH/CO in the present experiment. The abundance of CO in interstellar ices was typically about 25% or less relative to H2O (Gibb et al. 2004), which leads to the OH/CO of 4 or higher. The Rmax for OH/CO of 4 is approximately 0.1. In the ISM, many reactions including photolysis and hydrogenation compete with reaction (3). Therefore, the estimation of OH/CO in the ISM presented here may have a large uncertainty. Nevertheless, the Rmax value of 0.1 is significant to conclude that reaction (3) is one of the important CO2 formation pathways in the ISM.
The importance of surface reactions for the formation of most solid components, with the exception of CO, in icy grain mantles has long been proposed by a number of theoretical studies (e.g. d'Hendecourt et al. 1985; Hasegawa et al. 1992). Recent experimental studies have demonstrated that surface reactions involving CO can yield other abundant solid species in ice mantles, such as H2CO and CH3OH, through successive hydrogenation (Hidaka et al. 2004; Watanabe et al. 2006). Furthermore, the most dominant solid molecule, H2O, can be formed through a successive hydrogenation to O2 molecules on a cold grain surface (Miyauchi et al. 2008; Oba et al. 2009; Ioppolo et al. 2008). Detailed results and discussion on these surface reactions are summarized in a recent publication by Watanabe & Kouchi (2008). The present study clearly shows that CO2, one of the most abundant molecules in ice mantles, was formed through surface reactions of the neutral species CO and OH, as well as energetic processes involving UV, ions, or electrons (e.g., Watanabe & Kouchi 2002). Although the formation of HCOOH was not positively confirmed in the present study, it is possible that surface reactions of HOCO with atomic H yield HCOOH (Goumans et al. 2008). Thus, we expect that a series of theoretical and experimental studies will facilitate a more complete understanding of reaction routes to formation of H2O and CO-related molecules observed in interstellar icy grain mantles.
We thank an anonymous referee for helpful comments on the manuscript. This work was partly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and by a research fellowship from JSPS for Young Scientists (Y.O.).