Abstract
The central nervous system (CNS) has a highly complex biophysical and biochemical environment. Despite decades of intensive research, it is still an enormous challenge to restore its functions and regenerate lost or damaged CNS tissues. Current treatment strategies remain sub-optimal because of (1) the hostile microenvironment created post CNS injury, and (2) insufficient understanding of the pathophysiology of acute and chronic CNS diseases. Two-dimensional (2D) in vitro models have provided tremendous insights into a wide range of cellular interactions. However, they fail to recapitulate the complex cellular, topographical, biochemical, and mechanical stimuli found within the natural three-dimensional (3D) CNS. Also, the growing ethical needs to use fewer animals for research further necessitates 3D in vitro models to mimic all or part of the CNS. In this review, we critically appraise the status quo and design considerations of 3D in vitro neural disease and injury models that resemble in vivo conditions. This review mainly focuses on the most recent advances in tissue engineering techniques such as microfluidics, organs-on-a-chip and stem cell technology. Furthermore, we review recent models aiming to elucidate the underlying pathophysiology of CNS diseases. If armed with deeper understanding, it will be possible to develop high-throughput drug screening platforms and new treatments for CNS diseases and injuries.
Export citation and abstract BibTeX RIS
1. Introduction
The central nervous system (CNS) is the most complex entity of the human body. It is susceptible to irreversible degenerative and traumatic injuries, which can severely impair its function and significantly reduce the life quality of patients. CNS diseases and injuries remain some of the most challenging medical, social, and economic problems to date [1]. Specifically, CNS diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) are significantly more prevalent in the elderly [2, 3]. With an ageing global population, the number of people affected by these degenerative neurological disorders is expected to rise to unprecedented levels. Therefore, laboratory-based, bottom-up studies of the complicated pathophysiological mechanisms of the CNS diseases promise to accelerate the evaluation and development of novel repair strategies.
To study CNS pathology, researchers have relied on animal models and in vitro 2D cell cultures. These two methods have revealed many fundamental findings including the roles of specific genes, molecular and cellular signalling pathways. However, they both suffer from various drawbacks: (1) The use of animals for research has raised ethical concerns from animal rights groups. (2) There is a wide gap between animal and human physiology, so one must always extrapolate animal data to predict the human scenario. (3) On the other hand, although 2D cell cultures circumvent ethical issues, they fail to provide a realistic CNS microenvironment and thus cannot fully recapitulate cellular behaviours or cell–matrix interactions. More recently, organotypic cultures modelling traumatic CNS injuries have gained popularity for enabling the simultaneous evaluation of different independent factors in the same animal, thereby considerably reducing time and costs associated with in vivo models. Comprising of a multi-cellular in vitro environment, organotypic cultures render the elucidation of the underlying mechanisms of injury as well as the evaluation of numerous treatments feasible. Compared to a 2D culture environment, organotypic conditions can, to a degree, bridge the gap between single/co-culture systems and in vivo models. However, there remain several challenges to further improve this system; (1) by its very nature, organotypic models exhibit axotomized neuronal pathways, which require reconstruction prior to exhibiting any functionality, (2) most organotypic brain slices to study diseases such as AD or PD are derived from embryonic or post-natal donors due to their superior survival, which, however, does not adequately reflect the adult characteristics of AD or PD, and (3) maintaining long-term culture conditions and sterility to study disease mechanisms and screen arrays of candidate drugs renders organotypic models relatively laborious and costly. Within a native tissue, cell–cell and cell–extracellular matrix (ECM) interactions establish a 3D communication network through biochemical and biophysical cues that is vital in maintaining the specificity and homoeostasis of tissues [4]. These interactions also regulate key events in the cell life cycle, such as proliferation, migration, and apoptosis [5]. Therefore, a 3D model based on human-derived cells that re-establishes in vivo cell–cell and cell–ECM interactions is superior to conventional 2D cultures and animal models.
Ideal 3D in vitro models not only incorporate the appropriate cell types and biomimetic ECM, but also provide biochemical (e.g. growth factors) and biophysical cues (e.g. mechanical stimuli). This will ensure greater precision and reliability when recreating the complex and intricate nature of the microenvironment encountered within native tissues [6, 7]. These extracellular cues can significantly influence cell viability, proliferation, migration, and differentiation within both the brain and the spinal cord [8–10]. Considering the great significance of the 3D CNS models, herein we will review the engineering efforts required to simulate various components of the native CNS microenvironment. First, various applicable techniques for mimicking in vivo CNS microenvironments are reviewed. Then, we discuss the importance of various biochemical and biophysical cues in the CNS, such as ECM, bioactive, architectural, mechanical and electrical cues. Lastly, we dedicate the bulk part of this review to evaluating the most recent 3D in vitro models of common CNS diseases and injuries. Towards the end, we outline our projections with respect to the future directions of engineering more precise 3D in vitro CNS models.
2. Techniques for engineering in vitro 3D cultures
To recreate microenvironmental cues found under in vivo conditions, various micro-scale techniques are currently available, including spheroids, organoids, electrospinning, microfluidics and 3D printing, etc [11–13]. Principally, these techniques can be either scaffold-free or scaffold-based. Recent reviews on the progress of micro-scale 3D brain modelling using various technologies can be found from [14–17].
2.1. Scaffold-free in vitro 3D culture
Scaffold-free models do not require a physical scaffold or matrix, as the cells can produce their own ECM. For example, spheroids are self-assembled spherical clusters of cell colonies and can closely mimic the 3D architecture of in vivo CNS tissues [18]. Due to easy synthesis, such technique has gained great popularity in modelling various CNS diseases [19]. Moreover, when co-cultured with other cell types, spheroids can model the in vivo intercellular signalling, architecture, and hence the complicated CNS pathophysiology [9, 20, 21]. For instance, Vadivelu et al used a floating liquid marble technique to generate uniform-sized spheroids consisting of olfactory ensheathing cells (OECs) [22]. The transplantation of the OEC spheroids can bridge the defect and promote axonal regeneration in spinal cord injuries [23]. By co-culturing OEC spheroids with Schwann cells and astrocytes, they observed unique cell characteristics that were unreported in 2D cultures.
Similarly to spheroids, organoids also do not require scaffolds to form. They are self-assembled aggregates of multiple cell types derived from pluripotent stem cells or isolated organ progenitors [24]. An organoid is a miniaturised, simplified in vitro model of an organ, and can accurately reflect some of the human brain microenvironments [25–27]. For instance, Ormel et al created cerebral organoids, within which microglia spontaneously developed with their characteristic ramified morphology. Upon inflammatory stimulation, the organoid-derived microglia had similar responses compared to their native counterparts [28].
Both the spheroid- and organoid-based 3D cultures possess great potential in modelling CNS diseases and are believed to be effective tools for high-throughput drug testing [16, 17]. However, their reliance on passive oxygen and nutrient diffusion severely limits their maximum size, which results in necrotic cores if surpassed [27].
2.2. Scaffold-based in vitro 3D culture
A scaffold can overcome the above-mentioned limitation by providing a pathway for nutrients and oxygen to reach the core. In addition, a scaffold can have tuneable bioactive, architectural, mechanical, and electrical cues. With these controllable parameters, one can engineer a unique tissue microenvironment resembling that found within the ECM of CNS tissues.
2.2.1. Electrospinning
Electrospinning is an easy, versatile and popular technique for producing tissue-engineered scaffolds. It creates fibres with ECM-like structures and enables one to easily tune physical properties such as fibre diameter and porosity [29, 30]. In a typical setup, a high voltage potential over the working distance creates an electrostatic force, which draws charged polymer threads to a collector. If the collector is a stationary plate, the deposited micro- or nanofibres form a mat with random orientations. On the other hand, a collector with a rotating wheel leads to fibres with orderly, parallel alignments. For neural tissue engineering, a uniaxially aligned fibrous network that orients cell growth is an important criterion to ensure accurate simulation [31, 32]. Xie et al found that the direction of neurite outgrowth can either be parallel or perpendicular to the nanofibre alignment, depending on parameters including fibre density, protein deposited on fibre surface, and surface properties of the supporting substrate [33].
In one study, Luo et al used electrospun polylactic acid (PLA) nanofibrous scaffolds for long-term culture of neuromuscular junction, a specialised synapse associated with neurodegenerative diseases. They co-cultured primary embryonic motor neurons from Sprague-Dawley rats and C2C12 cells in a random or aligned nanofiber configuration. While the conventional 2D glass substrate could only maintain the culture for 2 weeks, random and aligned PLA scaffolds had 7-week cell survival rates of 55% and 70% respectively [34].
In another study, Jakobsson et al used a semi-spherical array of metal needles as the collector for electrospinning. Without the compression of fibres in a typical setup, they obtained low-density electrospun poly-ε-caprolactone (PCL) fibrous scaffolds. The high porosity allowed for full 3D infiltration of neural cells. Using neural progenitor cells, they observed a highly integrated network, synaptogenesis, and extensive neurite outgrowth. Compared to the 2D culture, where neuronal cells grew on top of glial cells in separate layers, cells intermixed in 3D culture, which is observed in vivo [35].
2.2.2. Microfluidics
Microfluidic technology tightly controls microlitres to picolitres of fluids within networks of micro-channels. Microfluidic chips are excellent in vitro models to study CNS degeneration and regeneration for three reasons [12]. Firstly, these platforms enable one to analyse cell secretions, transcriptions, and protein expressions at the single-cell level through designed compartmentalisation. As a result, one can study more in-depth with regards to myelination, neurite outgrowth, signal propagation, and neuronal networks [36, 37]. Secondly, such platforms can help probe cell–cell communication by co-culturing different CNS cells in one or more connected chambers [38–40]. Thirdly, microfluidics can accurately model or control the biophysical and biochemical cues of the microenvironment, so that one can monitor various cellular events post-injury or post-disease [41].
For example, Wevers et al used a microfluidic platform to culture induced pluripotent stem cells (iPSCs)-derived neural stem cells (NSCs) into 3D, ECM-embedded, neuronal-glial networks [42]. The platform had a 384-well microtiter plate format, with 96 tissue chips (4 wells per chip). The plated organoids were suitable for 3D analyses of biological processes such as cell differentiation, cell–cell interactions, cell–ECM interactions and related gene expression. Similarly, Wang et al created an organ-on-a-chip system to mimic blood brain barrier (BBB) for drug screening. They used human induced pluripotent stem cells to produce brain microvascular endothelial cells, and cocultured them with rat primary astrocytes. They used this microfluidic system to test permeability of various model drugs, and obtained data that were comparable to in vivo values [43].
2.2.3. 3D printing
3D printing is a technique that deposits materials in a layer-by-layer fashion to create 3D objects. With the help of computer-aided design software, one has the capacity to produce customisable hardware parts for rapid prototyping of CNS disease models. For instance, Johnson et al printed microchannels and compartmentalised chambers using PCL. They then deposited neurons, Schwann cells, and epithelial cells into separate chambers based on how cells are organised in vivo. The team proceeded to obtain insights about pseudorabies virus infection, thus highlighting the usefulness of this nervous system on a chip [44].
As a branch of 3D printing, 3D bioprinting utilises biological ingredients (e.g. biomaterials incorporated with viable living cells) as the ink to build functional 3D tissue constructs [45]. With precise spatiotemporal control over cell and biomaterial distributions, 3D-bioprinted objects can have accurate, complex and even personalised features that imitate the fine shape and architecture of natural tissues [46, 47]. While this technique has shown promising results in treating a range of brain-related injuries and disorders, it also possesses the capacity to produce normal or diseased tissues for cell behaviour studies [48]. For example, Lozano et al printed brain-like structures with discrete layers as an in vitro model to study cortical cell survival and axonal development [49]. Specifically, primary cortical neurons were encapsulated within a peptide-modified hydrogel, gellan gum-RGD to create a three-layer construct that included two cellular layers (top and bottom) and an acellular middle layer. After culture for 5 d, they observed that a neuronal network was formed in the brain-like structure, and extensive axons penetrated into the acellular layer. These results not only validated the versatility of bioprinting to control cell and ECM organisation for constructing a complex and viable 3D cell-containing construct, but also highlighted the possible reproduction of a more accurate 3D in vitro brain-like microstructure that might increase our understanding of neurological diseases and injuries.
2.3. Stem cell technology
Current disease and injury models mainly use primary cultures of CNS cells (e.g. NSCs, neurons, astrocytes, oligodendrocytes) dissociated from embryonic or early postnatal tissues of mice, rats, or adult neural cells [8]. Well-established difficulties extrapolating results derived from animal cells and the phenomenon of senescence associated with adult stem cells have fuelled the need for better alternatives that have a greater potential in mimicking human CNS disease states. One promising strategy involves harvesting recent exciting developments in human stem cell technology and integrating this knowledge into sophisticated engineered artificial 3D microenvironments to generate realistic CNS-like platforms to study damaged or diseased neural tissues. In particular, iPSCs represent a revolutionary technology to obtain 3D in vitro cell-based tissue equivalents. iPSCs can be generated using readily accessible cells (e.g. human fibroblasts) from patients with any condition and then be differentiated into disease-relevant neural cell types through numerous reliable protocols [50, 51]. Although there are still some roadblocks to consider, an in vitro disease model with an iPSC-based technology has many advantages. The most distinct one being that patient-specific iPSCs carry the precise genetic profile that may result in relevant diseases in the respective individual, accurately recapitulating disease phenotypes and providing an enviable opportunity to study complex genetic diseases of the CNS, especially in the case of rare diseases [11, 52]. Additionally, these disease models are able to elucidate the mechanisms of action by studying the initial development and pathological progression, as well as predicting patient treatment responses, which can pave the way for personalised regenerative medicine using the patient's own cells [52]. For example, iPSCs derived from PD patients, would not only represent a more powerful tool in replicating PD in vitro and deciphering its pathophysiological mechanisms, but could also further provide a source for replacement therapies [53]. In addition, stem cell technology can be combined with other above-mentioned scaffold-free or scaffold-based techniques to model a more brain-like 3D in vitro microenvironment.
3. Design considerations for recapitulating natural microenvironment in the CNS
The native CNS microenvironment is profoundly complex and is governed by a complex interplay between its 3D matrix, cellular components, naturally occurring signalling moieties, growth factors and cytokines. Novel advances in the microtechnological manipulation of tissue engineered 3D scaffolds focus on the mimicry of biochemical and biophysical cues found within the native CNS microenvironment (figure 1). The following subsections will summarise the most recent advancements in tissue engineering of the environmental cues in the context of the latest microtechnologies used to obtain realistic 3D in vitro microenvironments.
Figure 1. Schematic illustration of tissue engineering strategies employed to model injuries and diseases of the CNS. Biophysical and biochemical cues are engineered into a 3D matrix by various microtechnologies and then integrated with different cell types. Using these 3D in vitro models, one can investigate underlying pathophysiological mechanisms, screen drug candidates, and develop platforms for personalised medicine.
Download figure:
Standard image High-resolution image3.1. Engineering CNS ECM
As an integral part of the CNS microenvironment, the ECM affects almost all aspects of the nervous system development and function (e.g. cell support, NSC maintenance, differentiation and behaviour of progeny cells) [54, 55]. Currently, both natural and synthetic materials have been developed to construct a scaffold that can mimic the ECM properties of the in vivo microenvironment. The diverse composition of the ECM in the CNS is distinct compared to those of other organs. It is largely composed of proteoglycans of the lectican/hyalectan-family and their binding partners hyaluronic acid, link proteins, and tenascins [56, 57]. For this reason, natural materials that are the native ECM compounds or polymers extracted from tissues can inherently display many bioactive elements. For example, decellularized ECM, one of most promising natural materials, has received increasing attention. Medberry et al used decellularised ECM derived from porcine brain and spinal cord to synthesise a hydrogel scaffold. They then proceeded to use this scaffold to support unipolar or bipolar neurite growth and extension [58]. In comparison to decellularised scaffolds derived from the urinary bladder matrix, the CNS-ECM scaffolds resulted in longer neurites. This finding illustrated the importance of providing a relevant microenvironment to suit a particular tissue engineering purpose.
Despite these benefits, natural materials are difficult to purify reproducibly, frequently resulting in batch-to-batch variability. In addition, they are structurally weaker and liable to be disrupted when the cells attach on them and apply a cell-generated tension. Therefore, synthetic materials have been successfully introduced to scaffold fabrication because of their excellent mechanical support and scaffold stability. However, they usually lack the necessary bioactive elements. To combine the advantages of both natural and synthetic materials, synthetic materials modified with various bioactive elements have been proposed [59–62]. The usage of different scaffold modification techniques to alter material characteristics grants a tighter control over the behaviours of exogenous stem cells and can serve to guide the behaviours of endogenous ones. Overall, given the complexity of the CNS in both health and ill-health, the ability to externally control the features of scaffolds mimicking CNS microenvironments to guide cell behaviour is of fundamental importance to further understand the mechanisms of CNS pathologies. This will ultimately result in the development of better treatment strategies.
In addition to the ECM components, the extracellular microenvironment contains a variety of cues that guide cellular behaviours and determine the cell fate. They can be divided into two categories: biochemical (e.g. peptides, growth factors, cytokines, cell–cell co-culture) and biophysical (e.g. architecture, mechanical and electrical stimuli).
3.2. Engineering biochemical cues
Bioactive moieties such as peptides and growth factors influence cell behaviours with cell–material interactions for specific and controllable responses [63]. A typical approach used in the designing of such bioactive materials is to chemically integrate ECM whole proteins or ECM peptide sequences into scaffold materials. The use of peptide sequences as opposed to whole protein conjugates is particularly favoured due to its simpler conjugation chemistry and lower cost. For example, Wang et al created a self-assembling nanofibre scaffold composed of RADA-16 peptides with alternating positively and negatively charged amino acid residues. The scaffold was modified with FG loop (FGL) motif that was the synthesised peptide ligand of fibroblast growth factor receptor derived from the neural cell adhesion molecule [64]. Using the unmodified scaffold as a negative control, they investigated how the FGL motif affects the behaviours and functions of rat spinal cord-derived NSCs [65]. The FGL-enriched scaffolds displayed better proliferation and migration into the 3D nanofibrous scaffolds, while maintaining similar levels of neural differentiation. Apart from peptides, the scaffolds can be modified with other bioactive agents such as neurotrophins [66, 67] and therapeutic drugs (e.g. chemotherapeutic compounds) [68, 69]. These agents can modulate the cellular environment both in vitro and in vivo, to accelerate neuronal growth and functional recovery after CNS injury.
Neurotransmitter-based materials are another type of bioactive scaffolds that can improve the cell–material interactions. Neurotransmitters such as dopamine and acetylcholine are chemical messengers secreted by neurons and are critical for modulating neural activity in the nervous system. Hence, integrating their functionalities into biomaterials may be a feasible alternative to guide axonal projections and promote neuronal growth [70–72]. Further details describing engineered bioactive cues for nerve tissue engineering can be found in other reviews [63, 73].
More recently, with the advances in microfluidic technology, researchers have developed organ-on-chip systems which manipulate bioactive cues. For instance, Kim et al used a compartmentalised microfluidic device to control axonal growth by both surface modification and soluble factors [74]. After making a laser-induced lesion, they analysed cell–cell interactions between neurons and glial cells. Moreover, the platform enabled them to examine the complex bidirectional signalling processes that occur in the specific neuronal structures including axons and dendrites.
3.3. Engineering biophysical cues
Scaffold architecture also plays a vital role in cellular responses such as neural development [75–79]. The ECM offers a natural network of nanofibres to support cells and to guide cell behaviour via focal adhesion interactions [80]. In neural tissue engineering, there is tremendous potential in developing scaffolds that imitate the architecture of natural human tissues at the micro- and nano-scale [81–85]. For example, Lee et al found that a larger diameter (2–4 μm) of electrospun polystyrene fibres tended to support myelination compared to smaller nanofibers (0.2–0.4 μm) [86]. Coating the smaller nanofibers with biopolymers such as laminin [87], nectin-like protein 1 (NECL1) [88], and poly(L-lysine) did not improve myelination, illustrating the strong influence of physical size. Mohtaram et al discovered that compared with the larger diameter (85 ± 4 μm) of electrospun poly (ε-caprolactone) fibrous scaffolds, fibrous scaffolds with a smaller diameter (43.7 ± 3.9 μm) could induce higher expressions of the neural markers (e.g. Nestin and Pax6) in iPSCs [89]. In addition, the aligned substrate topography can influence neurite outgrowth [90, 91] and NSC differentiation [92, 93]. Bechara et al found NSCs had better cell attachment, proliferation, and elongated morphology, when grown on micro-patterned nanowire surfaces bordered by surfaces without a structured topography [94]. Li et al designed a high-throughput, microfluidic screening device with a large library of micro-patterned substrates. They assessed how those topographical features can act as physical cues to promote neuronal development including axon and dendritic outgrowth [95].
In addition to the fibre diameter, the aligned substrate topography has also been proven to influence neurite outgrowth [90, 91] and NSC differentiation [92, 93]. In addition to the geometric features at the nanoscale level, the higher level of organisation of the substrate at the microscale level also proved to be important for engineering NSC microenvironments [94, 96]. For example, in a study by Bechara et al, NSCs grown on micro-patterned nanowire surfaces bordered by surfaces not exhibiting a structured topography exhibited improved cell attachment, proliferation and elongated morphologies [94]. Similarly, another study by Thapsukhon et al showed that when nanofibrous sheets were rolled into a tube to guide neural development, improved cell attachment and proliferation were observed [97]. The tubular shape provided adequate cell binding and increased permeability, which allowed for cell infiltration and fluid and nutrient diffusion. These findings indicate that there is greater potential for nanofibrous tubes to be used as temporary scaffolds in reconstructive nerve surgery.
Besides scaffold architecture, cells also respond to surrounding mechanical stimuli via membrane receptors, which can regulate their morphology, proliferation, and differentiation [96, 98]. Brain tissue is one of the softest tissues in the human body and its elastic modulus is about 1 kPa, with some variation depending on age and anatomical location [99, 100]. In one study, Leipzig grew forebrain-derived stem cells on a photo-polymerizable methacrylamide chitosan biomaterial with tuneable Young's elastic modulus [101]. The results showed that stem cells proliferated optimally on 3.5 kPa substrates and that they differentiated into mature neurons when the Young's modulus of the substrate was <1 kPa. Keung et al further explored the molecular mechanisms through which stem cells could transduce mechanical cues to determine cell fate [102]. The study used Rho-family guanosine triphosphatases (Rho GTPases), the extensively studied molecular switches that regulate a wide range of signal transduction pathways in somatic cells. They found that Rho GTPases enabled NSCs to adjust their own stiffness in response to substrate stiffness. Consequently, the NSCs could selectively differentiate into either astrocytes or neurons. Another study found that increasing contact stiffness from physiological values (100 Pa) to shear moduli (≥10 kPa) can lead to morphological and inflammatory changes of both primary rat microglial cells and astrocytes, in vitro and in vivo. In addition to matrix stiffness, applied in vitro mechanical force such as strain, compression and shear can trigger various cellular responses [103, 104]. For example, Chang et al found that for neurites grown on parallel channels, stretching caused the neuronal marker β-tubulin III to rise and the expression of MAP2 to increase significantly. These findings indicated that mechanical tension may not only promote NSCs to differentiate towards neuronal cells, but also enhance neurite outgrowth and its maturation [105]. Overall, passive and active mechanical cues both play a vital role in the physiological process of CNS cells.
Electrical activity also has a remarkable influence on both the CNS development and its regenerative processes post-injury [106]. There are many findings demonstrating that exogenous electrical stimulations (ESs) play a significant role in modulating neural behaviour [107–109]. For example, ESs at a physiological level may regulate and expedite the directed migration of NSCs towards the cathode [110, 111]. The migration directedness and distance to the cathode increased with increasing field strength, whilst reversal of the field polarity reversed the migration [112]. Another study by Aznar-Cervantes et al proved that ESs are superior over neural growth factor treatments in causing PC-12 cells to differentiate into cells with neural phenotypes [113]. Depending on the voltage, the electrical field gradient can also affect adult NSCs, both morphologically and phenotypically. A direct current with short-duration (<10 min d−1 for 2 d) ESs on NSCs in vitro combined with biochemical factors resulted in mature neuronal morphologies and signs of differentiation [114]. The neurite lengths were evidently longer compared to those with no stimulation. Additionally, the neurites and soma of neuronally differentiated NSCs displayed elevated levels of calcium ions during stimulation, indicating the presence of functional neurons with the ability of electrical conductance and communication with other cells.
CNS disease/injury models that combine both biochemical and biophysical cues can closely mimic their native counterparts [106]. In the following sections, we will comprehensively review the latest developments of 3D in vitro CNS models created by various microtechnologies.
4. 3D in vitro disease and injury models of CNS
4.1. Neurodegenerative disease models
4.1.1. Alzheimer's disease
AD is the most common form of dementia and is characterised by a gradual decline in cognitive and executive functions. AD affects 5.5 million people in the United States alone and with an ageing population, cases are predicted to increase dramatically [115, 116]. There is no curative treatment, and symptomatic management is limited [117–119]. The two major pathological hallmarks of AD are β-amyloid plaques and the accumulation of neurofibrillary tangles [120, 121]. The 'amyloid hypothesis' posits that the excessive accumulation of β-amyloid peptides results in neurofibrillary tangles composed of hyperphosphorylated tau, which subsequently accumulates in axons, dendrites, and cell bodies. These will ultimately cause neuronal death [122–124]. This hypothesis has only recently been proved in an in vitro setting. Commonly used cell models do not reflect the complexity of AD as it is still challenging to simultaneously incorporate various cellular components (e.g. axons, synapses) and AD-specific pathological proteins (e.g. tau) [125].
3D cultures can recapitulate the natural CNS microenvironment to promote neuronal maturation and increase tau formation, which is essential for reconstituting tauopathies such as AD [126]. In one study, Zhang et al cultured neuroepithelial stem cells derived from human iPSCs, with RADA-16 self-assembling peptides (SAPs). Upon adding cations, the SAPs self-assembled into a 3D hydrogel (figure 2(A)) [127]. Both P21-activated kinases (PAKs; a critical link in mechano-transduction pathways) and drebrin (an actin-stabilising protein in the brain) had high degrees of expressions in these 3D hydrogels, but not in 2D culture models. They postulated the reason to be related to the soft mechanical surroundings (stiffness of a few Pascal), which resembles brain tissue much more than conventional 2D culture dishes. The addition of Aβ oligomers, which are thought to be directly linked to the pathogenesis of AD [128], attenuated the expressions of both proteins. This was observed in the 3D SAP matrix, but not in 2D culture systems, further proving the superiority of the 3D model [100]. However, it did not evaluate the aforementioned 'amyloid hypothesis' by investigating whether excessive accumulation of Aβ oligomers would lead to neurofibrillary tangles composed of aggregated tau proteins.
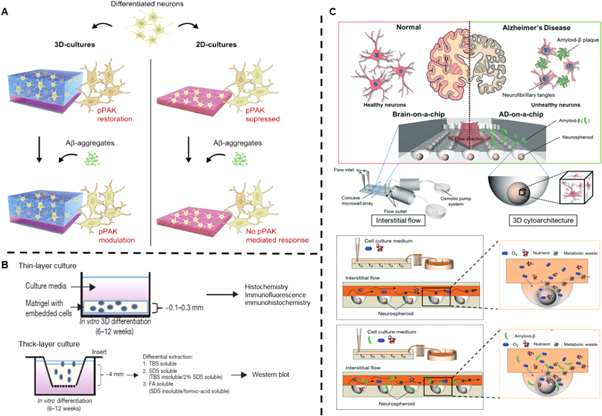
Figure 2. 3D neural cell culture models for AD. (A) Schematic diagram illustrating the differences of pPAK expression of differentiated neuron and its responses to Aβ oligomers in 2D cultures and 3D SAP matrix cultures. Reproduced from [127], Copyright 2014, with permission from Elsevier. CC-BY-NC-ND 3.0. (B) Schematic diagram of the thin-layer and thick-layer 3D culture protocols as well as detergent extraction processes. Reproduced with permission from [129]. (C) The structural design of a 3D brain-mimicking microfluidic device. Normal and AD brain-mimicking microfluidic platform with neurospheroids cultured under dynamic conditions without or with addition of synthetic amyloid-β, respectively. Reproduced from [130], Copyright 2015, with permission from The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageSubsequently, Choi et al provided experimental validation of this hypothesis (figure 2(B)) [129]. In their study, they used ReNcell VM human neural precursor (ReN) cells embedded in a Matrigel scaffolds and supplemented with neuronal growth factors. They first demonstrated the accumulation of high levels of both β-amyloid and phosphorylated tau. Then, they attenuated tauopathy by inhibiting β-amyloid generation. The findings thus provided a possible causal link between these two pathological processes. The scaffold material had high levels of brain ECM proteins, providing appropriate bioactive and architectural cues for optimal cell differentiation. Furthermore, compared to 2D culture conditions, 3D culture displayed dramatically increased neuronal and glial differentiation, β-amyloid deposition and subsequent levels of tau isoforms. The authors postulated the reason to be bioactive cues and secreted β-amyloid diffusing into a large volume of media in 2D conditions with subsequent removal upon media changes. A 3D culture, on the other hand, would limit diffusion and thus accelerate neuronal and glial differentiation and aggregation of β-amyloid.
Microfluidics aims to enhance current 3D disease models to create in vivo-like dynamic microenvironments. In one study, Park et al used a 3D culture-based microfluidic device as an AD model, and exposed neurospheroids to interstitial flow. Compared to static cell cultures, the dynamic culture conditions yielded larger spheroid sizes, had a greater neurite extension and an enhanced neural progenitor cell differentiation. This was likely due to the continuous supply of fresh medium and removal of metabolic waste through the flow. Additionally, exposure of neurospheroids to β-amyloid by an osmotic micropump remarkably decreased their viability compared to when they were exposed under static conditions (figure 2(C)) [130]. The reason was believed to be the interstitial flow enabling deeper infiltration of β-amyloid into the neurospheroids, causing more neurons to undergo apoptosis. The addition of dynamic flow further adds to a more realistic in vitro brain model. This model was further enhanced by the same group to include microglial cells capable of inducing a neuroinflammatory microenvironment [131]. Using a microfluidic triculture system involving neurons, astrocytes and microglia resulted in microglial recruitment and secretion of neurotoxic soluble factors with subsequent loss of neurons and astrocytes. It was identified that chemotactic microglial recruitment depended on Aβ accumulation and upregulation of chemokine (C–C motif) ligand 2/monocyte chemoattractant protein 1 (CCL2/MCP-1), both factors are known to be upregulated in human AD brains.
Despite recent advances, the design of 3D in vitro models must account for some important limitations. For instance, the self-assembling nature of some 3D AD models makes them difficult to control, resulting in marked differences in cell microenvironments [127] and batch-to-batch variations [132]. Furthermore, the use of iPSC-derived neurons to model AD holds several limitations: (a) their relative developmental age does not consider the fact that AD usually develops in adults beyond their fifth decade [133], and (b) the variability within iPS cell lines and resulting genetic and epigenetic variations of subsequent clones make it difficult to develop reproducible in vitro models [134].
4.1.2. Parkinson's disease
PD is the second most common neurodegenerative disorder, characterised by bradykinesia, rigidity, resting tremor, and deteriorating cognitive function [135]. An abnormal accumulation of the α-synuclein protein in the form of toxic Lewy bodies results in irreversible degeneration of dopamine-producing neurons originating in the substantia nigra pars compacta. PD has no cure, and current treatment options are mainly limited to dopamine replacement strategies, which are complicated by long-term movement-related side effects [136, 137]. Understanding the complex molecular pathophysiology of PD, it is crucial to developing effective therapies. However, it is nearly impossible to extract live neurons from PD patients, let alone make representative in vitro models to study the disease [138].
Stem cell-based in vitro models mimicking PD have thus been developed using cell cultures and synthetic scaffolds [139]. For example, iPSCs derived from PD patients have been differentiated into dopaminergic neurons exhibiting signs of PD pathophysiology in vitro. Up until recently, most in vitro studies have relied on 2D cultures to demonstrate dopaminergic differentiation of stem cells. This, as previously described, fails to fully mimic the microenvironment in a living organism. As a result, 3D SAP scaffolds composed of amino acid sequences have been developed to improve survival and differentiation of NSCs [140, 141]. SAP scaffolds could support cell viability and induce much higher differentiation of murine embryonic stem cells into dopaminergic neurons, compared to 2D plates (41.5% ± 3.4% versus 8.3% ± 1.4%) [142]. This was thought to be due to the differences in spatiotemporal distribution of nutrients, cell surface receptors and other molecular signals in 3D culture environments. In particular, a higher surface exposure to midbrain patterning factors in the 3D SAP system was considered to be the crucial differentiating factor between 2D and 3D systems.
Although animal-derived stem cells are useful, their experimental results cannot be fully translated into human setting. Brito et al cultured human midbrain-derived neural progenitor cells (hmNPCs) of foetal origin. Under stirred culture conditions, the hmNPCs aggregated into neurospheres and differentiated towards a dopaminergic lineage [143]. The same group further developed this dynamic culture system for more efficient dopaminergic differentiation and neuronal maturation, making it suitable as a 3D in vitro PD model [144]. They exposed their neurospheres to the bioactive signalling factor cyclic adenosine monophosphate (cAMP). This led to significantly upregulated dopaminergic markers since cAMP is thought to promote the differentiation, maturation, and survival of midbrain dopaminergic neurons [145, 146]. These studies demonstrate the relevance of 3D in vitro neurospheres in elucidating PD pathogenesis. By taking advantages of neuronal precursor cells to self-organise into 3D structures [19, 147], one can mimic the fundamental processes of brain development [148]. This provides significant advantages in recapitulating physiological and pathophysiological mechanisms of brain function and dysfunction. Nevertheless, the foetal origin of the neural progenitor cells limits their clinical relevance in modelling PD associated with old age.
4.1.3. Multiple sclerosis (MS)
MS is a chronic inflammatory disorder of the CNS, characterised by immune cell infiltration, demyelination, axonal degeneration, and gliosis [149]. Myelin is essential for the efficient transmission of action potentials throughout the nervous system. Following demyelination, for unknown reasons, only limited remyelination occurs [150], characterised by a smaller diameter of myelin [151]. It is therefore crucial to develop reproducible in vitro models to study how demyelination happens, and what biochemical and biophysical cues can induce better remyelination in MS.
For instance, Harrer et al exposed murine organotypic cerebellar slice cultures (OSCs) to demyelinating factors to characterise their in vitro regenerative abilities [152]. This ex vivo system was found to be useful for evaluating therapeutic strategies to prevent demyelination and enhance remyelination in MS patients. However, disadvantages like loss of myelin during staining and questionable data caused by insufficient diffusion of demyelination-inducing agents through whole brain slices remained. For these reasons, Vereyken et al developed 3D whole brain spheroid aggregates in vitro, in which all cell types of the CNS were represented [153]. They exposed the single cell suspensions of rodent embryonic brains to constant rotational forces on a gyratory shaker. These continuous mechanical cues supported the spherical aggregation of embryonic brain cells into 3D spheroids. Once cultured for 4 weeks, myelin formation was detected throughout the whole spheroid. Exposure of spheroids to lysophosphatidylcholine (LPC), an agent used to induce demyelination [154], led to isolated myelin breakdown while sparing axons and causing little astrogliosis. They achieved remyelination thanks to OPCs present within whole brain spheroids, which provide mitogenic cues [155] and trophic factors [156] that were required for myelin production and unaffected by LPC. Furthermore, LPC toxicity could be attenuated by compounds such as simvastatin, which supports process extension and OPC differentiation. These results demonstrate that this 3D in vitro model can model demyelination and investigate interventional strategies.
4.1.4. Huntington's disease (HD)
HD is an incurable hereditary neurodegenerative disorder, affecting around 5–7 individuals out of 100 000 (lower in Asian countries) [157]. It is inherited in an autosomal dominant fashion, typically manifesting between the ages of 35–55 with changes in personality, cognition, and motor skills [158]. The clinical course of HD is progressive for over many years, ultimately leading to severe brain atrophy and death [159]. For individuals affected with HD, there is an expansion of CAG repeat region within the huntingtin (HTT)-encoding genes, resulting in aggregates of polyglutamine [160].
Many stem cell-based 2D in vitro HD models have been developed [161–163], but a limited number of relevant physiological models exist. Similarly, animal models, for the aforementioned reasons, are increasingly falling out of favour. Therefore, researchers have developed more suitable in vitro systems to elucidate HD pathophysiology. For example, Zhang et al used suspensions of self-aggregating HD-iPS cells to generate NSCs [164]. Within this 3D system, the HD-NSCs differentiated into striatal neurons containing the same CAG expansion found in the HD patient from whom the iPS cell line was established. Such differentiated cells could serve as a human HD cell model to analyse its pathophysiology or for drug screening. Despite some success in developing stem cell-based 3D in vitro cultures, most knowledge about the molecular pathways of HD still comes from analyses of HD mouse models or post-mortem HD tissue. With the help of patient-derived iPSCs, new avenues of elucidating the pathophysiology of HD will be available in the future.
4.2. Traumatic injury models
Traumatic brain injury (TBI) is amongst the most serious public health problems worldwide [165]. It is usually caused by an external physical impact resulting from falls, sports injuries, motor vehicle accidents, and explosions [165]. Currently, there are very few effective treatments available [12]. This is because the CNS has a limited regenerative capacity due to the lack of Schwann cells in the peripheral nervous system. Moreover, glial scar tissue containing nerve growth inhibitory factors acts as a mechanical and biochemical barrier for both axon growth and myelination [166].
In vitro models of CNS trauma are typically obtained by using different mechanical stimuli, such as compression, stretch, and laceration. For example, Bar-Kochba et al identified the effects of impact strain and strain rate on primary cortical neurons embedded in collagen gels (figure 3(A)) [167]. In another study, Weightman et al used a modified scalpel to sever an organotypic spinal cord slice and create an in vitro spinal cord injury (SCI) model. They then examined cell-nanomaterial interactions in this injury-simulating environment (figure 3(B)) [168]. This model not only replicated cellular responses to in vivo neurological injury, but also demonstrated that aligned topography could induce the outgrowth and alignment of astrocytes and neurons within injury sites. In another study, Zuidema et al observed a similar effect of topography on cells following SCI. They seeded astrocytes and neurons on an anisotropic-to-isotropic electrospun poly-L-lactic acid (PLLA) fibre/film, which resembled the SCI-induced structural changes [169]. They showed that neurite outgrowth was aligned on the fibrous parts of the biomaterial, but reduced when approaching the isotropic, non-fibrous domains.
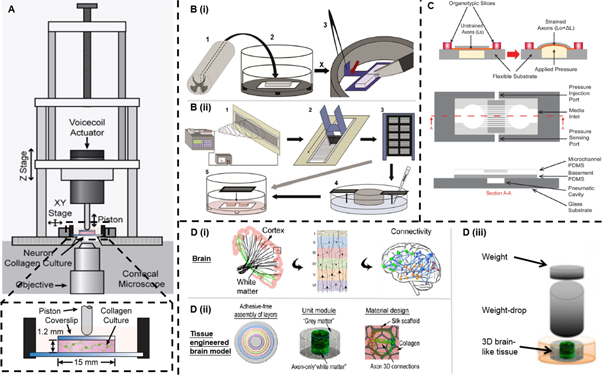
Figure 3. 3D in vitro CNS traumatic injury models. (A) Schematic diagram of neuronal compression model fitted onto a confocal microscope for spatiotemporal nerve injury analysis. Reproduced from [167], CC BY 4.0. (B) Schematic diagram of (i) the production of organotypic lesioned spinal cord slice cultures and (ii) the fabrication of the aligned electrospinning nanofibers to cover the lesioned slices. Reproduced from [168], Copyright 2014, with permission from Elsevier. (C) Schematic diagram (before and after strain application, top and sectioned views) of the organotypic uniaxial axonal strain model. Reproduced from [170], Copyright 2013, with permission from The Royal Society of Chemistry. (D) Schematic diagram of the 3D brain-like cortical tissue model. (Di) The architectural features of the brain. (Dii) The design concept of the modular brain-like tissue model. (Diii) The design of TBI model to study the brain-like tissue responses by the weight-drop impact on the tissue. Reproduced from [171], Copyright 2014, with permission from PNSA, Proceedings of the National Academy of Sciences.
Download figure:
Standard image High-resolution imageMore recently, Dolle et al used a microfluidic device to model diffuse axonal injury, one of the most common pathological features of TBI (figure 3(C)) [170]. In the device, they cultured organotypic brain slices on a polydimethylsiloxane substrate. This model could precisely control the strain on individual axons or bundles of axons in a 3D environment. After applying external pressure to cells, they observed axonal responses that are typically seen in vivo following human brain injury. This model enables repeated testing and is a non-invasive research platform.
To further engineer functional 3D in vitro brain-like cortical tissues, Tang-Schomer et al designed a compartmentalised model. They seeded primary neurons in a porous silk scaffold and filled the space with a collagen gel (figure 3(D)) [171]. The scaffold-gel composites demonstrated mechanical properties comparable to rodent brain tissue and maintained the neural culture for months. They used a weight-drop model to induce TBI and demonstrated impact force-dependent injury responses. Furthermore, the model exhibited excitatory neurotransmitter glutamate release, and impact-induced transient hyperactivity which was similar to in vivo conditions.
4.3. Neurodevelopmental disorder models
4.3.1. Epilepsy
Epilepsy encompasses a variety of syndromes which predispose the affected individual to generating an abnormal, transient discharge of neurons in the brain, or a seizure [172]. Depending on the brain region involved, the effects of seizures range from changes in cognition, convulsions and unusual sensations. Realistic in vitro disease models of epilepsy are useful for toxicity testing of new pharmacological agents and elucidating underlying mechanisms of seizure generation. Additionally, in vitro models enable the discovery of side effects of the tested drugs early on, thus increasing efficacy and reducing cost. Unfortunately, current models heavily rely on expensive animal models, rendering their results possibly untranslatable to the human scenario. Ideally, an in vitro model of epilepsy should employ cell types found in the human brain capable of forming functional neurophysiological networks and displaying seizure-like activity. It should further be amenable to high-throughput drug screening [173]. Currently available in vitro seizure models including their advantages and limitations are summarised in table 1.
Table 1. Comparison of in vitro seizure models (Reproduced from [173]. CC BY 4.0.).
| Model | Advantages | Limitations |
|---|---|---|
| Acute slice assay | √ Validated representative of in vivo adult rodent brain | × Difficult inter-species extrapolation |
| √ Defined cytoarchitecture | × Damage to cytoarchitecture and neuronal projections during preparation | |
| √ 'Gold standard' | × Low throughput | |
| Organotypic slice culture | √ Representative of in vivo rodent | × Difficult inter-species extrapolation |
| √ Retains functional tissue networks | × Neonatal source, so not representative of matured system | |
| × Time consuming | ||
| Primary CNS culture | √ Representative of in vivo cell types | × Difficult inter-species extrapolation |
| √ Validated model | × Loss of 3D structure | |
| √ Higher throughput than slices | × Not representative of multicellular architecture of in vivo CNS | |
| iPSC-derived culture | √ Human-based, humanoid pheno- and genotype, can model genetic component of epilepsy of patients with specific mutations | × Expensive and time consuming |
| √ Fewer ethical considerations | × No standard protocol |
4.3.2. Autism
Autism spectrum disorder (ASD) is a complex and heterogeneous neurodevelopmental disorder, which is usually defined as a cluster of symptoms including deficits in social communications, interactions, and restricted, repetitive patterns of behaviours. In some cases, ASD can cause cognitive delay. Little is known about the pathophysiology of ASD [174]. Animal models still cannot fully recapitulate such disorders, due to the broad spectrum of behavioural phenotypes and the inherent difficulty in recreating human-like behavioural patterns in rodents. During differentiation of normal human neural progenitor cells, many ASD-related genes and signalling pathways are highly co-expressed indicating the neurodevelopmental origin of ASD [175, 176]. Thus, relevant models should focus on the early disease development during which genes and environmental factors may play a significant role.
In a recent study, Mariani et al developed 3D neural cultures in organoids derived from iPSCs to explore neurodevelopmental variations in patients with severe ASD [177]. While they did not identify a single underlying genomic mutation, gene network analyses showed an upregulation of genes involved in cell proliferation, neuronal differentiation, and synaptic assembly. Organoids developed from individuals with ASD displayed an accelerated cell cycle and overproduction of GABAergic inhibitory neurons, which are hypothesised to be an underlying cause of ASD.
Rett syndrome (RTT), while not technically part of ASD according to symptomatology, shares commonalities with ASD in its early stages [178, 179]. Maria et al isolated fibroblasts from patients with RTT symptoms, and infected them with retroviral reprogramming vectors [180]. After culture for 2–3 weeks, compact iPSC colonies appeared in the background of fibroblasts, and then were manually picked up and transferred to Matrigel. They dissociated the cell clusters and plated them onto low-adherence culture dishes for 5–7 d to obtain embryoid bodies (EBs) for neural differentiation. The formed EBs were then transferred and plated on poly-ornithine/laminin-coated dishes. After a week of culture, EB-derived rosettes became visible and were collected for use. The RTT-iPSCs maintained the ability to undergo X-inactivation and generate proliferating NSCs and functional neurons. This model has the potential to recapitulate the early stages of some neurodevelopmental diseases. It may also be a promising tool for disease diagnosis, drug screening, and personalised treatment for RTT and ASD.
Thus far, EBs are one of the most common methods of modelling neurodevelopmental disorders in vitro. However, it remains questionable whether these models can reflect neurodegenerative diseases accurately enough to draw clinically relevant conclusions. Much more research is required to progress from the current stages of infancy to technically mature models.
4.3.3. Microcephaly
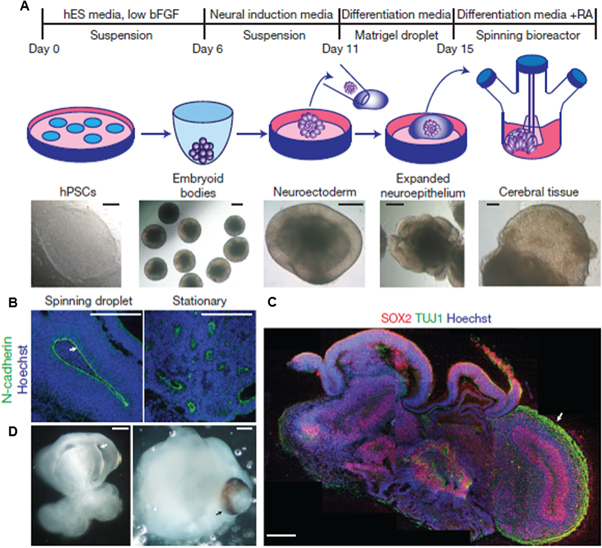
Microcephaly is another neurodevelopmental disorder characterised by a reduction in brain size. Currently, the underlying causes and mechanisms of microcephaly are poorly understood [181], and there is still no available treatment. To address this issue, human iPSC-derived cerebral organoids have been applied in the study of microcephaly [27, 182]. For example, Lancaster et al developed an in vitro model using fibroblast-derived iPSCs taken from a patient with severe microcephaly [27]. These iPSCs self-assembled into 3D EBs and were embedded into droplets of Matrigel to provide an in vivo-like scaffold (figure 4). Continuous spinning acted as a mechanical cue to ensure optimal nutrient absorption, resulting in the rapid development of brain tissues within 20–30 d. Compared to control EBs, those derived from microcephalic iPSCs demonstrated smaller neural tissues with only a few progenitors, and exhibited premature differentiation into neurons. This phenotype could be reversed by reintroducing CDK5RAP2, a protein that causes premature neuronal death when mutated and is associated with microcephaly. This model recapitulates some fundamental mechanisms of mammalian neurodevelopment and could be used in the future to develop interventions to prevent the development of microcephaly in utero.
Figure 4. (A) Schematic demonstration of the 3D cerebral organoids cell culture system. (B) Neuroepithelial tissues produced by this technique. (C) Immunohistochemical stains of neural progenitors (SOX2, red) and neurons (TUJ1, green) showing complex, heterogeneous regions of cerebral organoids. (D) Reminiscent ventricles (fluid-filled cavities) shown with white arrow and retina tissue shown with black arrow. Scale bar: 200 μm. Reproduced with permission from [27].
Download figure:
Standard image High-resolution image4.4. Other disease models
Migraine is a common neurological disorder characterised by moderate to severe headaches, typically with throbbing or pulsating sensations which can significantly reduce quality of life. It usually lasts from a few hours up to a day, heavily inhibiting the productivity of patients [183]. Cortical spreading depression (CSD), which is considered to be the physiological mechanism behind the migraine aura, is a propagating wave of large-scale grey matter depolarisation. To better understand it, Tang et al developed a CSD model using a microfluidic platform and mouse organotypic brain slices [184]. Through precise focal control of the chemical stimuli (potassium ions) in different areas of the cortical layers, they found that CSD may be induced under conditions related to brain damage and induce states such as migraine.
Another CNS disorder called neuronal migration disorder usually occurs when developing neurons are unable to migrate to the appropriate areas within the brain. Possible causes include genetic mutations and deletions of genes, which cause microtubules and actin-associated proteins to denaturate, disrupting the accurate cortical patterning in the cytoskeleton development [185]. It is possible to study the genotype-phenotype correlations between mutated genes and neuronal migration disorder by observing how neurons arise from NSCs and migrate to the CNS [186]. More recently, Bamba et al created a disease model using iPSCs derived from the cerebral cortex of a lissencephaly patient. They used the serum-free embryoid body-like aggregate method to develop brain-like structures in floating culture [187]. This model recapitulated the pathogenesis of human neuronal migration disorder and enabled the team to observe the real-time behaviour of human cortical neurons for a long time.
Friedreich's ataxia (FRDA) is another type of pathogenic mutation, and is caused by a transcriptional defect in the frataxin gene [188]. Atrophy of sensory and cerebellar pathways results in ataxia, dysarthria, unstable fixation, loss of deep sensory and tendon reflexes, later leading to a heightened risk of diabetes and death-inducing cardiomyopathy [189]. To understand how FRDA develops, Hick et al isolated neural precursors and cultured them in suspension to form neurospheres. The neurospheres extended in all directions and formed a dense network over a month, thus mimicking the process of neural development [190]. FRDA iPSC-derived neurons showed not only GAA expansion instability but also signs of mitochondrial functional damage.
Last but not least, schizophrenia (SCZ), a severe psychiatric disorder, is characterised by delusions, hallucinations, social withdrawal, cognitive deficits, and loss of emotion and motivation [191]. Currently, iPSC-derived neurons from the fibroblasts of schizophrenic patients are the gold standard for developing SCZ models [192–194]. da Silveira Paulsen et al used such a model to study oxygen metabolism in SCZ, and correlated SCZ development with changes in the levels of oxygen consumption and reactive oxygen species [194]. Despite some unresolved limitations, the iPSC-derived disease models are predicted to provide further insight into the molecular and cellular underpinnings during the initiation and progression of SCZ.
5. Conclusion and future prospective
The extracellular microenvironment is tremendously important in controlling the behaviour of CNS cells. In vitro models that mimic the natural microenvironment will enable one to study CNS pathology or effects of potential medications. In this review, we have outlined the typical parameters required to recreate CNS microenvironments including ECM, biological, architectural, mechanical and electrical cues. We have also described the commonly used techniques to engineer the abovementioned cues. Lastly, we have reviewed various CNS disease models that researchers have developed.
To date, most CNS disease and injury models have been designed primarily to elucidate known or suspected mechanisms, or to validate isolated observations. In addition, no single device has the capacity to completely reconstruct the in vivo CNS environment. Besides the challenge of incorporating every single cell type in their respective representative numbers, the realistic reconstruction of a functional 3D CNS microenvironment is commonly limited by a lack of or delayed vascularisation of the core of the 3D construct. This, in turn, increases the risk of core necrosis and system failure. It becomes more and more evident that a multi-dimensional approach to tissue engineering is required to further understand the intricate workings of such microenvironments. Future efforts to engineer 3D CNS microenvironments will require a combination of different technologies while mimicking multiple aspects of the native CNS microenvironment. For in vitro and ex vivo tissue cultures, they are most likely to comprise a designer ECM scaffold inoculated with NSCs or their progeny cells, as well as a second, or even third, cell microenvironment component. In addition, strategies to accelerate 3D vascular network formation, such as employing 3D scaffolds with prefabricated tubular networks could assist in rapid generation of realistic CNS microenvironments. With continuous advances, we envision that future CNS disease models will make it possible to fully elucidate specific mechanisms and to identify new treatment strategies.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (11702233) and the start-up fund (1-ZE7S) and central research fund (G-YBWS) from the Hong Kong Polytechnic University.