Abstract
Post-operative isoflurane has been observed to be present in the end-tidal breath of patients who have undergone major surgery, for several weeks after the surgical procedures. A major new non-controlled, non-randomized, and open-label approved study will recruit patients undergoing various surgeries under different inhalation anaesthetics, with two key objectives, namely (1) to record the washout characteristics following surgery, and (2) to investigate the influence of a patient's health and the duration and type of surgery on elimination. In preparation for this breath study using proton transfer reaction time-of-flight mass spectrometry (PTR-TOF-MS), it is important to identify first the analytical product ions that need to be monitored and under what operating conditions. In this first paper of this new research programme, we present extensive PTR-TOF-MS studies of three major anaesthetics used worldwide, desflurane (CF3CHFOCHF2), sevoflurane ((CF3)2CHOCH2F), and isoflurane (CF3CHClOCHF2) and a fourth one, which is used less extensively, enflurane (CHF2OCF2CHFCl), but is of interest because it is an isomer of isoflurane. Product ions are identified as a function of reduced electric field (E/N) over the range of approximately 80 Td to 210 Td, and the effects of operating the drift tube under 'normal' or 'humid' conditions on the intensities of the product ions are presented. To aid in the analyses, density functional theory (DFT) calculations of the proton affinities and the gas-phase basicities of the anaesthetics have been determined. Calculated energies for the ion-molecule reaction pathways leading to key product ions, identified as ideal for monitoring the inhalation anaesthetics in breath with a high sensitivity and selectivity, are also presented.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The monitoring of unlabelled drugs in the body in real-time offers an opportunity to determine their therapeutic effectiveness and washout characteristics in a continuous and non-invasive way. By way of illustration, several soft chemical ionisation studies have highlighted the use of breath analysis to detect intravenous anaesthetics, such as propofol during surgery [1–5], or to monitor the concentrations of inhaled anaesthetics post-surgery, either in the breath of patients or within operating and recovery rooms in hospitals [6–10]. The former has led to the development of a low cost analytical device to monitor propofol levels in the breath of patients during anaesthesia or under sedation in real-time [11]. The latter provides interesting information on the length of time these anaesthetics remain in a patient's body so that the pharmacokinetics of these anaesthetics can be better understood. Within this context, the work presented in this paper explores some potential applications of breath gas analysis in inhalation anaesthesia, which are applicable to use in a clinical setting.
In a recent proton transfer reaction—quadrupole—mass spectrometric (PTR-Quad-MS) study, involving a number of severely ill patients who had undergone major surgery, it was found that the inhalation anaesthetic used, isoflurane, took several weeks to be fully eliminated [10]. This was a very limited study, involving a small number of extremely sick people, and hence a key aim of our new research programme is to extend this pilot isoflurane study to explore the elimination characteristics for a number of inhalation anaesthetics used in surgical procedures, and to investigate the dependence of the lifetime of an anaesthetic in the body on various factors that could influence the outcome, including the duration and type of surgery, the quantity of an anaesthetic inhaled, ventilation, and the health, body mass index (BMI), gender, and age of patients.
Once inhaled, an inhalation anaesthetic enters the blood stream through the alveoli where it is transported and delivered to the brain, but on its journey to the brain some of the anaesthetic becomes stored in tissues. Of the inhalation anaesthetics being investigated, desflurane and sevoflurane have the lower tissue solubility, and therefore we can expect those two anaesthetics to be eliminated from the body in shorter timescale than that for isoflurane. Nevertheless, their lifetime in the human body may still be long given that they are primarily eliminated from the body via exhalation, and are only minimally metabolised by the liver and kidneys [12]. The low solubility of sevoflurane in blood suggests that this agent should enter and leave the body more rapidly than isoflurane. The closeness of sevoflurane and isoflurane tissue/blood partition coefficients suggests that the rates of equilibration with and elimination from tissues should be similar [13]. Yet sevoflurane is eliminated faster than isoflurane, despite its greater blood/tissue partition coefficient, but slower than desflurane.
The Medical University of Innsbruck has recently granted us ethical approval to undertake long-term respiratory gas analyses of patients following scheduled surgery. For this, the patients will be classified according to their American Society of Anaesthesiologists (ASA) physical status classification, which is used for defining the pre-operative risk assessment of the physical conditions (fitness) of patients [14]. Our study is restricted to ASA 1–3, where ASA 1 refers to normal healthy individual, ASA 2 denotes pre-operative patients who have a mild systemic disease and ASA 3 is assigned to those patients who have a severe systemic disease. It will be of considerable interest to see how the physical fitness and physical status of our volunteers will affect the retention and exhalation of an inhalation anaesthetic.
For the analyses of breath samples, we will be using mass spectrometric analytical methods, with the key instruments being proton transfer reaction mass spectrometry (PTR-MS) and ion mobility spectrometry. Proton transfer reaction mass spectrometry is a popular analytical tool used for detecting volatile compounds in complex chemical environments for a wide range of applications, ranging from food sciences through to homeland security [15–20]. It is particularly ideal for real-time measurements, which is useful for tracking rapid changes of trace volatiles such as occurs in the atmosphere, industrial processing and in breath and for those studies for which pre-preparation and pre-concentration of the gas samples are not possible.
Before any detailed breath washout measurements on any analytical device are undertaken, it is important to ascertain how the various anaesthetics can be best detected (highest sensitivity with high selectivity) for a given analytical instrument. In the case of PTR-MS this is associated with determining the product ions (types and intensities) resulting from the reactions of reagent ions with the individual anaesthetics in the drift (reaction) tube over a range of reduced electric fields. (The reduced electric field is defined by the ratio E/N, where E is the electric field strength and N is the total molecular gas number density in the drift tube of a PTR-MS. The unit used for E/N is the Townsend (Td), where 1 Td = 1 × 10−17 V cm2.)
In the PTR-Quad-MS study of isoflurane (C3H2ClF5O, monoisotopic mass (lightest isotopomer) 183.97 Da) by Fernández del Río et al [10], the product ion branching percentages over a small range of reduced electric fields (96–138 Td) were investigated. Prior to that study, PTR-Quad-MS studies of isoflurane were reported by Rieder et al [6] at one fixed E/N to determine the air quality in a post-anaesthetic care unit. In that study they claim to have monitored protonated isoflurane, but no such product ion was observed in the study by Fernández del Río et al. Only product ions resulting from dissociative proton transfer are reported in the paper by Fernández del Río et al. Therefore, a minor objective of the work presented in this paper is to resolve this discrepancy in the observed product ions between the two published isoflurane studies.
In addition to isoflurane, two other halogenated ether compounds are routinely used as modern inhalation anaesthetics for surgical procedures, namely desflurane (C3H2F6O, 168.00 Da) and sevoflurane (C4H3F7O, 200.01 Da). The choice of anaesthetic is determined by the anaesthetist on its pharmacological properties, a patient's underlying diseases and use of medication, the type of breathing system being used, and duration and type of surgical procedure [12].
The first reported PTR-MS study of sevoflurane is by Critchley et al [21], using the same PTR-Quad-MS as used in the later isoflurane study of Fernández del Río et al but only at one fixed reduced electric field of approximately 140 Td. As found for isoflurane, proton transfer to sevoflurane is dissociative. Given the low mass resolution of PTR-Quad-MS systems, the reported product ions are only listed at nominal m/z values of m/z 199, tentatively identified as C4F7O which would result from the elimination of H2 from the protonated parent, m/z 181, identified as C4F6H2OH+, which results from the loss of HF from the protonated parent, and m/z 49, ascribed to be CHFOH+. The product ion at m/z of 181 was by far the dominant product ion observed by Critchley et al. In agreement to this, Summer et al [8], who also used a PTR-Quad-MS instrument, report using a product ion at m/z of 181 to monitor sevoflurane, although it is incorrectly identified in their work as the protonated parent. In a later study by Trefz et al [9], who used a Proton Transfer Reaction—Time-of-Flight—Mass Spectrometer (PTR-ToF-MS), which has a far higher mass resolution compared to a PTR-Quad-MS, they reported sevoflurane product ions at m/z ratios of 181.007 and 198.999, with the product ion at m/z 181.007 being by far the most intense, in agreement with the study by Critchley et al. Given the high mass accuracy achievable with a PTR-ToF-MS, the product ion reported by Trefz et al at m/z 198.999 is certainly consistent with the product ion assignment, C4F7O
which would result from the elimination of H2 from the protonated parent, m/z 181, identified as C4F6H2OH+, which results from the loss of HF from the protonated parent, and m/z 49, ascribed to be CHFOH+. The product ion at m/z of 181 was by far the dominant product ion observed by Critchley et al. In agreement to this, Summer et al [8], who also used a PTR-Quad-MS instrument, report using a product ion at m/z of 181 to monitor sevoflurane, although it is incorrectly identified in their work as the protonated parent. In a later study by Trefz et al [9], who used a Proton Transfer Reaction—Time-of-Flight—Mass Spectrometer (PTR-ToF-MS), which has a far higher mass resolution compared to a PTR-Quad-MS, they reported sevoflurane product ions at m/z ratios of 181.007 and 198.999, with the product ion at m/z 181.007 being by far the most intense, in agreement with the study by Critchley et al. Given the high mass accuracy achievable with a PTR-ToF-MS, the product ion reported by Trefz et al at m/z 198.999 is certainly consistent with the product ion assignment, C4F7O of Critchley et al.
of Critchley et al.
In contrast to the PTR-MS results, two investigations of the reaction of H3O+ with sevoflurane by Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) (Critchley et al [21] and Wang et al [22]) observed the same three product ions. In the study by Critchley et al the product ion at the nominal m/z value of 199 was by far the most intense of the three product ions. The earlier SIFT-MS study by Wang et al found that the intensities of the product ions varied greatly with the experimental parameters and conditions, namely reaction length and the humidity in the flow tube, respectively, with the longer the reaction length resulting in CHFOH+ being the dominant product ion. When using moist air in the flow tube, Wang et al observed that all of the product ions, other than CHFOH+, react with water and are lost.
Although enflurane is rarely used in operations, it is included in this study because it is still commercially available, and furthermore being an isomer of isoflurane it is analytically challenging to distinguish from isoflurane and provides an interesting comparison to the ion chemistry for isoflurane. To our knowledge, there have been no other reported PTR-MS studies of desflurane and enflurane.
Details of the current PTR-ToF-MS studies of the four inhalation anaesthetics, desflurane, sevoflurane, isoflurane and enflurane as a function of reduced electric field and drift tube humidity will be presented in the following text. These provide a new or an improved understanding of the ion-molecule chemistry occurring in the drift (reaction) region of a PTR-MS for these anaesthetics. This study clearly establishes which product ions and drift tube conditions should be used in order to monitor these anaesthetics in breath following surgery with a high selectivity and sensitivity. Furthermore, and for the first time, we present in this paper density functional theory (DFT) calculations of the proton affinities, gas-phase basicities and changes in the enthalpy and free energy for the key reaction pathways leading to the product ions to be monitored for all four of the halogenated ethers. These are used in this paper to aid in the interpretation of the observed product ions.
2. Methods
2.1. Chemicals
For these present studies, surgical grade inhalation anaesthetics were obtained. Sevoflurane (CAS number: 28523-86-6) and isoflurane (CAS number: 26675-46-7) were purchased from the biopharmaceutical company AbbVie, enflurane (CAS number: 13838-16-9) was supplied by Abbott Products Operations AG, and desflurane (CAS number: 57041-67-5) was sourced from Baxter International. The chemicals were used directly without further purification for the PTR-MS headspace analyses reported in this paper. All of these chemicals are liquids at room temperature.
2.2. Proton transfer reaction-time-of-flight-mass spectrometry (PTR-ToF-MS)
Thorough descriptions of the instrument's operating principles and details of its applications are provided in depth by Ellis and Mayhew [15]. Hence only pertinent details will be provided here. All of the PTR-ToF-MS measurements presented in this paper were taken using an Ionicon Analytik GmbH (Innsbruck, Austria) PTR-TOF 8000, with or without the addition of a multicapillary column (MCC) for gas chromatographic pre-separation. The MCC was used under 'normal' drift tube conditions to help in identifying the product ions coming from an anaesthetic of interest through limited pre-separation, thereby providing us with a higher confidence in product ion assignment to an anaesthetic. However, MCC separation capabilities are less than can be obtained with conventional (single capillary column) GC. For MCC, temporal resolution is sacrificed for smaller size and shorter cycle times, and therefore we cannot completely rule out an impurity that may be present in the sample being investigated contributing to the observed product ions.
The PTR-TOF 8000 and the MCC have been described elsewhere by Ruzsanyi et al [23], and therefore only a brief overview of settings is given here. The ion source was operated at a current of 3.5 mA and a voltage of 160 V, with the source-out voltage maintained at 140 V. The source valve opening was set at 40%. The drift tube's pressure and temperature were maintained at 2.3 mbar and 60 °C, respectively. The voltage drop across the drift tube was varied from 365 V up to 965 V at the fixed drift tube pressure, resulting in a range of reduced electric field values ranging from approximately 80 Td up to about 210 Td. The MCC was used at a temperature of 40 °C with a N2 (99.9999% purity) flow of 10 ml min−1. For the MCC measurements, resulting products ions under three reduced electric field values of 80 Td, 140 Td and 180 Td were investigated.
The mass spectra, obtained by converting the drift times of the ions in the ToF-MS analyser, ranged from approximately m/z 3 to m/z 230, and were acquired in a time of 1 s by co-adding 25 000 single 40 μs extraction periods recorded at a sampling frequency of 10 GHz. The mass resolution in the present experiment obtained from the detected peaks was ≈2400 at m/z 100. The total duration of a complete reduced electric field experimental run was approximately 14 min, which corresponds to the acquisition of 60 mass spectra per single E/N value. The averages of the ion signal levels at each m/z value identified to be associated with a given anaesthetic from these 60 spectra were used to calculate the intensities and branching percentages of the product ions.
Data were analysed using the PTR-MS Viewer 3.2.8, which also performed mass calibration and peak identification. Raw product ion signal intensities (counts per second) were used and, where needed for determining the sensitivity of detection, these were normalised to a constant reagent ion signal of 106  per second for each reduced electric field measurement. The
per second for each reduced electric field measurement. The  signal intensity cannot be measured directly, owing to its high intensity, which saturates the detector. Instead the signal intensity for the spectral line peaking at m/z 21.02, corresponding to
signal intensity cannot be measured directly, owing to its high intensity, which saturates the detector. Instead the signal intensity for the spectral line peaking at m/z 21.02, corresponding to  was recorded, from which the intensity of
was recorded, from which the intensity of  was calculated.
was calculated.
Two humidity conditions in the drift tube were investigated, which will be referred to as 'normal' or 'humid', with the latter approximately corresponding to that of exhaled breath. 'Normal' corresponds to the case where a dry buffer gas (absolute humidity <0.1%) is being flowed into the drift tube, but note that owing to diffusion of water vapour from the ion source into the drift tube, the buffer gas in the drift tube is not dry and hence is at a higher absolute humidity. 'Humid' refers to the situation when water saturated buffer gas is used (absolute humidity 5%).
Although H3O+ (or the protonated water dimer—depending on the value of the reduced electric field applied) dominates the reagent ion signal, other reagent ions are always present in the drift tube, with the dominant ones being NO+ and  which are at levels of 1%–2% and 2%–6% relative to H3O+, respectively, with the value depending on the reduced electric field value and under 'normal' operating conditions. Generally, at such low percentages they can be ignored, but care must be taken that product ions are not coming from reactions with these reagent ions if proton transfer from H3O+ or H3O+.H2O is not efficient or at values of E/N where the reagent ion signal intensities are low.
which are at levels of 1%–2% and 2%–6% relative to H3O+, respectively, with the value depending on the reduced electric field value and under 'normal' operating conditions. Generally, at such low percentages they can be ignored, but care must be taken that product ions are not coming from reactions with these reagent ions if proton transfer from H3O+ or H3O+.H2O is not efficient or at values of E/N where the reagent ion signal intensities are low.
2.3. Sampling procedures
The samples were prepared as follows: A Tedlar® bag (SKC Inc., USA) of 3 l capacity was flushed with N2 (99.9999% purity) multiple times in order reduce any possible background volatile contamination. Then the bag was filled with 2 l of dry N2 (for the so-called 'normal' measurements) or with 2 l of N2 bubbled through water at room temperature (for the so-called 'humid' measurements). Background mass spectra were recorded for each reduced electric field from approximately 80 to about 210 Td in steps of 10 Td, first ascending and then descending.
To prepare the anaesthetic samples, a 1 l gas bulb (Supelco, Canada) was maintained at 60 °C while evacuating. The gas bulb was then closed, and 0.5 μl of an anaesthetic was injected into it through a septum. Dry N2 was then introduced into the bulb to bring it to atmospheric pressure. Finally, a 5 ml sample of the gas was taken from the bulb and introduced into the bag containing either dry or humid N2. This bag was then connected to the heated inlet of the PTR-TOF 8000 for direct measurement.
The anaesthetic concentrations entering the drift tube were in the range of approximately 400–500 parts per billion by volume (ppbv). These concentrations are sufficiently low to ensure there was not any detectable loss of the reagent ion signal, but sufficiently high to give good product ion signal intensities.
2.4. Density functional theory calculations
Density functional theory (DFT) calculations have been undertaken to determine the proton affinities (PA) and gas-phase basicities (GB) of water, the water dimer, desflurane, sevoflurane, isoflurane and enflurane. These calculations were conducted using the Gaussian09W program with the GaussView05 for Windows interface and the B3LYP functional with 6–31 + G(d,p) basis set [24] at 298 K. It should be noted that using 298 K is arbitrary as the temperature of the drift tube bath (buffer) gas is greater than this (in this study it is at 333 K) and that the translational temperature of the ions even higher due to the electric field (see later). But this is not considered too important as the energetics (particularly ΔGs rather than ΔHs) are being used to assist in determining the likely fragmentation pathways to the observed ions [25].
A full account of the DFT results and their interpretation for the four anaesthetics is on-going, and are too extensive to be given with any detail in this experimentally focused paper. Furthermore, given that the main focus of this paper is to provide details on which product ion(s) should be monitored to determine the concentrations of these four volatile anaesthetics in breath with the best sensitivity, we only present sufficient DFT information to provide details on the pathways leading the key product ions that are to be recommended for monitoring purposes in the breath analysis research programme. A more detailed report on the DFT calculations will be the subject of another paper.
3. Results
3.1. PTR-ToF-MS results
3.1.1. H3O+.(H2O)n (n = 0 and 1) reagent ions
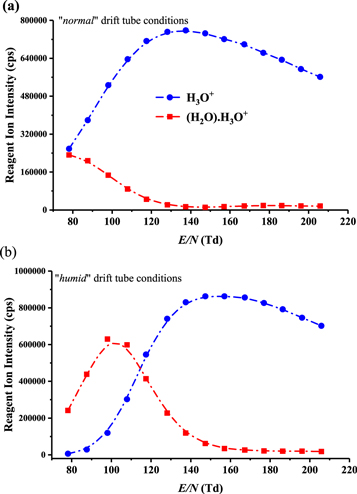
The intensities (counts per second) of the reagent ions H3O+.(H2O)n (n = 0 and 1) in the PTR-ToF-MS experiments, as measured at the detector as a function of reduced electric field (obtained by changing the drift tube voltage at constant drift tube pressure) under the 'normal' and the 'humid' drift tube conditions already defined [26, 27] are illustrated in figures 1(a) and (b), respectively. It can be seen that under 'normal' operating conditions, H3O+ remains the dominant reagent ion for the complete range of the reduced electric fields investigated. This is not the case under 'humid' conditions, for which the dominant reagent ion becomes H3O+.(H2O) for reduced electric fields below about 110 Td, and by about 80 Td it is the only reagent ion with any significant intensity.
Figure 1. Reagent ion intensities in counts per second (cps) for H3O+.(H2O)n, where n = 0 and 1, recorded at the detector of the PTR-TOF 8000 used in this investigation under (a) 'normal' and (b) 'humid' operating conditions as a function of the reduced electric field (E/N).
Download figure:
Standard image High-resolution imageUnder both 'normal' and 'humid' operating conditions we find that the reagent ions H3O+.(H2O)n (n = 2 and 3) make negligible contributions to the total reagent ion signal at any reduced electric field. For example, at the lowest reduced electric field applied (80 Td), under 'normal' and 'humid' conditions, combined they contribute less than 3% and 10%, respectively, to the total reagent ion signal, with their intensities significantly declining with increasing reduced electric field, as is expected. Although it should be appreciated that these intensities correspond to those measured at the detector and hence they may not be a completely true reflection of the distributions present in the drift tube, we have found that changes in voltage supplied to the ion extraction lens, which is situated behind the skimmer cone just after the exit of orifice of the drift tube does not significantly alter the relative intensities. Therefore, we will assume that the measurements at the detector provide a reasonable indication of the protonated water and protonated water cluster distributions within the drift tube. Given their low intensities under any operating conditions, the reagent ions H3O+.(H2O)n (n = 2 and 3) have been neglected in any analysis of the measurements obtained.
3.1.2. MCC-PTR-ToF-MS results
The m/z values (lightest isotopomer), the molecular ion formulae (the identification of which is greatly aided by the accurate measurement of the mass spectral peak owing to the high mass resolution of the PTR-TOF 8000) and distributions of the product ions (percentages) at three E/N values selected determined using the MCC-PTR-TOF 8000 are provided in table 1. The molecular formulae of the product ions have been identified via the exact m/z (to 2 decimal places) and isotopic (13C and where relevant 37Cl) intensities. This section only presents the product ions that have been identified to derive from a given anaesthetic and which result in a branching percentage of 3% or above at any given reduced electric field value investigated in this study. It should, however, be noted that there are difficulties associated with the accurate determination of the branching percentages owing to m/z discrimination in PTR-MS. There are several factors influencing this, including diffusional and Coulombic losses of the product ions in the drift tube, and m/z discrimination in the transfer optics, mass analyser and the ion detector. Collectively, they cannot be easily quantified with a reliable accuracy over a wide range of reduced electric fields, and thus reported branching percentages are only indicative rather than quantitative, and are rather specific for the PTR-MS and its settings being used.
Table 1. Product ion distributions (percentages) determined using the MCC PTR-TOF 8000 resulting from the reactions of the H3O+.(H2O)n (n = 0 and 1) reagent ions in the drift tube with the four halogenated ethers at three reduced electric field (E/N) values measured under 'normal' drift tube conditions. Only product ions which contribute at least a branching percentage of 3% at any given reduced electric field over the range of 80–210 Td have been included. The m/z values given for the neutral species and the product ions are for the lightest isotopomer, but the percentages provided have taken into account the contributions to the total ion signal of all isotopic variants (13C and where relevant 37Cl) to provide a more accurate branching percentage.
| Anaesthetic | Product Ions | E/N (Td) | ||
|---|---|---|---|---|
| Stochiometric Formula m/z (lightest isotopomer) | formula, m/z (lightest isotopomer) | 80 | 140 | 180% |
| Desflurane | C3H2F5O+, 149.00 | 87 | 56 | 25 |
| CF3CHFOCHF2 | C3F5O+, 146.99 | 8 | 20 | 6 |
| 168.00 | C2F4H+, 101.00 | — | 2 | 8 |
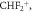 51.00 51.00 |
5 | 22 | 61 | |
| Sevoflurane | C4H3F6O+.H2O, 199.02 | 7 | 1 | 1 |
| (CF3)2CHOCH2F | C4H3F6O+, 181.01 | 68 | 97 | 81 |
| 200.01 | CF3+, 69.00 | — | — | 3 |
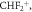 51.00 51.00 |
— | 2 | 15 | |
| CH2FO+, 49.01 | 25 | — | — | |
| Isoflurane | C3F4ClO+.H2O, 180.97 | 3 | — | — |
| CF3CHClOCHF2 | C3H2F4ClO+, 164.97 | 74 | 5 | 2 |
| 183.97 | C3F4ClO+, 162.96 | 8 | 6 | 1 |
| C3HF3ClO+, 144.97 | 5 | 1 | — | |
| C2HF3Cl+, 116.97 | — | 27 | 19 | |
| C2H2F2ClO+, 114.98 | 6 | 1 | — | |
| CHFCl+, 66.98 | 3 | 42 | 63 | |
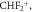 51.00 51.00 |
1 | 18 | 15 | |
| Enflurane | C3F4ClO+, 162.96 | 4 | 1 | — |
| CHF2OCF2CHFCl | C3HF3ClO+, 144.97 | 7 | 1 | — |
| 183.97 | C2HF3ClO+, 132.97 | 4 | 1 | — |
| C3H3F3Cl+, 130.99 | 31 | — | — | |
| C2HF3Cl+, 116.97 | 1 | 12 | 9 | |
| C2H2F2ClO+, 114.98 | 3 | 2 | 1 | |
| C2F2ClO+, 112.96 | 9 | 7 | 1 | |
| CHFCl+, 66.98 | 31 | 62 | 78 | |
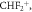 51.00 51.00 |
9 | 14 | 11 | |
Details of the various product ions follow in the next section. Here we comment that the proton transfer reaction of H3O+ with all four anaesthetics is found to be completely dissociative, with a substantial number of product ions resulting from the reaction, and this is particularly so for isoflurane and enflurane.
3.1.3. Reduced electric field (E/N) investigations of the product ion intensities resulting from the individual anaesthetics
The key aim of this paper is to provide details on what product ion(s) should be monitored and under what operational conditions for maximum sensitivity for detecting an inhalation anaesthetic in exhaled breath. Hence, following the identification of the product ions for all four anaesthetics using the MCC-PTR-TOF 8000, for each anaesthetic the intensities (counts per second) of the product ions were then monitored over a range of reduced electric fields without the MCC, under 'normal' and 'humid' drift tube operating conditions.
'Humid' measurements are crucial, because these are closer to breath samples, and it is known that humidity in the drift tube can affect the product ion distributions. This is highlighted in two recent studies, which investigated the product ion distributions for nine deuterated compounds [27] and a series of ketones [28] in the PTR-TOF 8000. For the deuterated study, with the exception of acetone-d6, the key effect of the higher drift tube humidity was an increase of the deuterium/hydrogen isotope exchange reactions of the primary product ions with the ever present water in the drift tube. This led to the generation of various isotopologue product ions. For the ketones, the effect of humidity was not observed to be so dramatic, with the general outcome of the higher humidity in the drift tube resulting in a need for higher E/N (approximately 20 Td higher) to obtain similar product ion intensities to those found under the 'normal' drift tube operating conditions
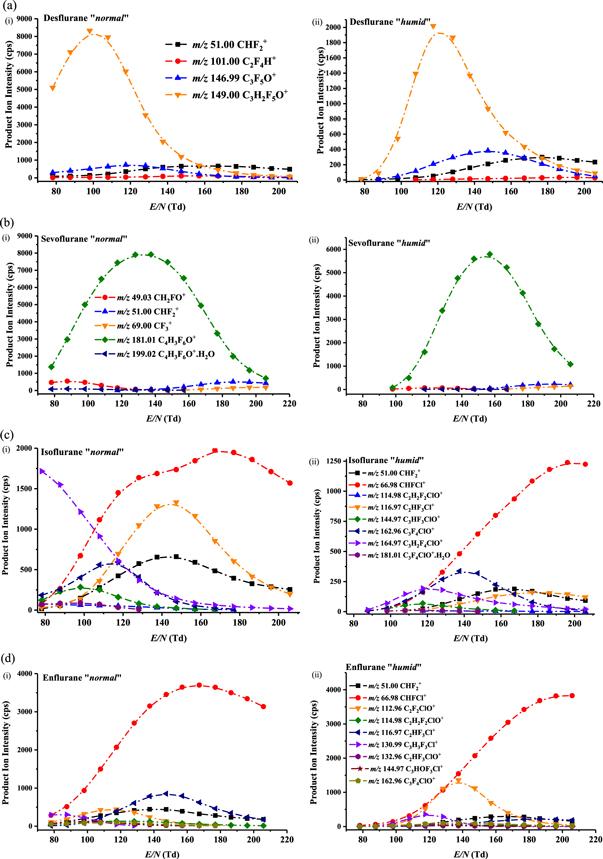
In comparison to the two earlier studies [27, 28], investigating the consequences of humidity on the product ion distributions, effects caused by differing humidity conditions in the drift (reaction) tube of a PTR-MS are found to be more dramatic on the production of the product ions for the anaesthetics studied in this investigation. Figure 2 provides the intensity curves (intensities of product ions in terms of counts per second (cps) as a function of the reduced electric field) for the product ions identified for (a) desflurane, (b) sevoflurane, (c) isoflurane and (d) enflurane over the range of reduced electric fields investigated under both (i) 'normal' and (ii) 'humid' drift tube conditions. What is noticeable from this figure immediately is the dramatic decrease in signal intensity under the 'humid' compared to the 'normal' drift tube conditions for low reduced electric fields (i.e. approximately <110 Td), and this is particularly so for isoflurane and enflurane.
Figure 2. Signal intensities in terms of raw counts per second of the product ions resulting from reactions of the H3O+.(H2O)n (predominantly n = 0 and 1—see figure 1 and text) with (a) desflurane, (b) sevoflurane, (c) isoflurane and (d) enflurane as a function of the reduced electric field (E/N) under (i) 'normal' and (ii) 'humid' drift tube operating conditions. The m/z values given for the product ions are for the lightest isotopomer, but the intensities have taken into account all isotopic variants, namely contributions from the 13C containing product ions and in addition, for isoflurane and enflurane, the 37Cl containing product ions.
Download figure:
Standard image High-resolution image3.1.3.1. Product ions resulting from desflurane and sevoflurane
For desflurane and sevoflurane the dominant product ion observed for the majority or all of the reduced electric field values investigated, respectively, results from the loss of HF following proton transfer from H3O+ to the anaesthetic. The difference in the E/N intensity profiles of this dominant product ion for desflurane and sevoflurane, figures 2(a) and (b), respectively, is surprising given that the same reaction pathway is followed with similar energetics. We have confirmed that this difference in intensity profiles is real by repeating the E/N intensity profile measurements with a dry and humid nitrogen sample containing a mixture of both desflurane (at approximately 500 ppbv) and sevoflurane (at approximately 400 ppbv).
For desflurane, above about 170 Td, the product ion at m/z 51.00 (assigned to be  ) becomes the dominant product ion. Other product ions originating from desflurane are at m/z values of 146.99 and 101.00, assigned to be C3F5O+ and C2F4H+, respectively, with their intensities being much less than the maximum reached by C3H2F5O+ at a lower reduced electric field.
) becomes the dominant product ion. Other product ions originating from desflurane are at m/z values of 146.99 and 101.00, assigned to be C3F5O+ and C2F4H+, respectively, with their intensities being much less than the maximum reached by C3H2F5O+ at a lower reduced electric field.
In addition to the dominant product ion, four other product ions have been assigned to result from the reaction of H3O+ with sevoflurane. One, at m/z 199.02, is considered to result from the association of water to the dominant product ion C4F6H2OH+. As found for desflurane,  at m/z 51.00, is also a product ion with a significant branching percentage, but with a relatively low intensity, at high reduced electric fields. The two other product ions observed are at m/z 69.00 (CF3+) and m/z 49.03 (CH2FO+), but with insignificant intensities compared to the maximum achieved by C4H3F6O+.
at m/z 51.00, is also a product ion with a significant branching percentage, but with a relatively low intensity, at high reduced electric fields. The two other product ions observed are at m/z 69.00 (CF3+) and m/z 49.03 (CH2FO+), but with insignificant intensities compared to the maximum achieved by C4H3F6O+.
Using a reduced electric field of 140 Td, a product ion resulting from the reaction of H3O+ with sevoflurane was identified with a nominal value of m/z 199 by Critchley et al [21]. This was detected with a higher accuracy at m/z 198.999 by Trefz et al [9]. As mentioned in the introduction, both studies assigned the product ion to be C4F7O In our measurements, at 80 Td the peak position in this mass range is at m/z 199.02, which is more consistent with the product ion C4F6H2OH+.H2O. It is possible that at higher reduced electric fields the product ion we observe at m/z 199 is indeed C4F7O
In our measurements, at 80 Td the peak position in this mass range is at m/z 199.02, which is more consistent with the product ion C4F6H2OH+.H2O. It is possible that at higher reduced electric fields the product ion we observe at m/z 199 is indeed C4F7O but its intensity is too low in our study for us to accurately measure its peak position. Given that, in agreement with this study, the two other PTR-MS studies that identify a product ion at a nominal m/z value of 199 show that the signal intensity relative to the other product ions is extremely low, it is of no relevance for determining breath concentrations of sevoflurane. Thus, given the low intensity involved, whether the ion at m/z 199 is either C4F6H2OH+.H2O and/or C4F7O
but its intensity is too low in our study for us to accurately measure its peak position. Given that, in agreement with this study, the two other PTR-MS studies that identify a product ion at a nominal m/z value of 199 show that the signal intensity relative to the other product ions is extremely low, it is of no relevance for determining breath concentrations of sevoflurane. Thus, given the low intensity involved, whether the ion at m/z 199 is either C4F6H2OH+.H2O and/or C4F7O is irrelevant to the current aim of this work, and hence we will not discuss it further in this paper.
is irrelevant to the current aim of this work, and hence we will not discuss it further in this paper.
3.1.3.2. Product ions resulting from isoflurane and enflurane
In comparison to desflurane and sevoflurane, for isoflurane and enflurane, the dominant product ion selected for monitoring purposes is at a much lower m/z value of 67, assigned to be CHFCl+, which can only occur via significant fragmentation. This is discussed further in section 3.2, which deals with the interpretation of reaction pathways with the aid of DFT calculations leading to the product ions to be monitored for the breath analysis programme. Given the number and complexity of the product ions observed for these two anaesthetics, a full discussion of the reaction pathways and the energetics involved will be the subject of another paper for which the full energetics will be provided. We will just comment here on one product ion observed for enflurane at m/z 130.99 (and at m/z 132.99 with an intensity one third of that at m/z 130.99), which is consistent with a product ion having a molecular formula C3H3F3Cl+. It is difficult to provide an explanation as to how this product ion can be formed, because it requires the elimination of F2O from the protonated parent.
3.1.3.3. Detection sensitivities of the anaesthetics
The 'humid' intensity curves provide the results needed to fulfil a key objective of this investigation, namely to determine the appropriate PTR-TOF 8000 operational parameters and the product ion(s) to monitor the four inhalation anaesthetics in exhaled breath with the highest sensitivity. The key results are summarised in table 2, which also provides details of the best sensitivity for 'normal' conditions for comparison. Table 2 and figure 2 show that under the 'normal' operating conditions, the best sensitivities are at lower reduced electric fields than required for a 'humid' drift tube.
Table 2. Best operating reduced electric fields values (highest sensitivity) and product ion to monitor for the anaesthetics desflurane, sevoflurane, isoflurane and enflurane in breath ('humid' drift tube conditions) and in closed environments (e.g. operating theatres) ('normal' drift tube conditions), determined using an Ionicon Analytik GmbH PTR-TOF 8000 instrument. The sensitivity of detection in terms of ncps/ppbv (total product ion cps normalised to 106 H3O+ reagent ions) under the 'normal' drift tube operating conditions are provided.
| Inhalation Anaesthetic | Required E/N (Td) for highest sensitivity 'humid' 'normal' | Sensitivity ncps/ppbv 'normal' | Key product ion(s) for monitoring an anaesthetic chemical formula m/z | |
|---|---|---|---|---|
| desflurane | 120 | 100 | 18 | C3H2F5O+ |
| 149.00 | ||||
| sevoflurane | 150 | 130 | 20 | C4H3F6O+ |
| 181.01 | ||||
| isoflurane | 200 | 150 | 11 | CHFCl+ |
| 66.98 + 68.98 | ||||
| enflurane | 200 | 130 | 9 | CHFCl+ |
| 66.98 + 68.98 | ||||
The total product ion intensity at the reduced electric fields for which the maximum signal is obtained to determine the sensitivity of detection in terms of normalised counts per second (ncps) (normalised to 106 H3O+ reagent ions) for 1 ppbv of an anaesthetic. The results for this are also presented in table 2.
We find that the sensitivity of detection for isoflurane and enflurane is less than that for desflurane and sevoflurane. Differences in reaction time will play a role, but also m/z dependent transmission factors will have an effect. In a TOF-MS there is a discrimination against lower m/z ions. Given these factors and measurement errors associated with the calculation of the concentrations, the sensitivities obtained are comparable, being between 10 and 20 ncps/ppbv.
For comparison, we investigated the instrument's sensitivity for detecting limonene, because it is known that limonene reacts with H3O+ with unit efficiency [29], and, given the proton affinity of (H2O)2, we can expect H3O+.H2O will react with limonene via a proton transfer process also at the collisional rate. For this compound the product ions to monitor are a fragment product ion at m/z 81.07, corresponding to C6H9+, and the protonated parent, C10H17+, at m/z 137.13. Given that the proton affinity of limonene is higher than that of water and the water dimer, the sum of the counts per second for the two product ions was normalised to 106 (H3O+ + H3O+.H2O). We find that we have maximum sensitivity for the detection of limonene at a reduced electric field of 80 Td, with a value of approximately 20 ncps/ppbv, i.e. similar to the sensitivities obtained for the anaesthetics.
3.2. DFT calculations
The experimental results show that for all four of the anaesthetics investigated in this study, a significant number of product ions are observed, and that all product ions are a result of dissociative processes, i.e. no protonated anaesthetic is observed. An objective of the computational calculations is to aid in interpreting this observation and to improve our understanding of the pathways leading to these product ions. These calculations have shown that many of the reaction pathways require significant increases in the free energy from that calculated at 298 K (up to several eVs in some instances) to deliver some of the product ions. The energy required to do this must be supplied to the ions through the translational energy gained in the electric field, which is in part converted to internal energy through collisions with the buffer gas. Within the drift tube thermodynamic equilibrium does not apply, because the reagent and product ions will have effective translational temperatures much higher than thermal owing to the drift velocities they obtain, which are dependent on ion mobility coefficients and the strength of the applied electric field. This results in collisional energies in the centre-of-mass frame between the reagent ions and the analytes that can drive reaction pathways, and once the primary product ions are produced resulting from the initial dissociative proton transfer reaction, these product ions can undergo further collisional induced dissociation.
Key to any analysis of product ions resulting from proton transfer are the values for the proton affinities (PAs) and gas-phase basicities (GBs) for the analytes. Table 3 presents the calculated PAs and GBs for water, the water dimer and for the four anaesthetics (various protonation sites are provided). To our knowledge, for the four anaesthetics investigated in this study, this is first time these thermodynamic quantities have been calculated and presented. The quoted energies for the key (dominant) reaction pathways are referenced to the flurane and H3O+ at 298 K.
Table 3. Calculated proton affinities (PA) and gas-phase basicities (GB) for water, the water dimer and for the four halogenated ethers in units of kJ mol−1. Calculations were performed using the B3LYP Functional and the 6–31 + G (d,p) basis set at 298 K. Values for a given anaesthetic are given for various protonation sites (see text).
| Compound | PA (kJ mol−1) | GB (kJ mol−1) |
|---|---|---|
| water | 684 | 653 |
| water dimer | 842 | 777 |
| desflurane | ||
| site 1 | 652 | 638 |
| site 2 | 623 | 621 |
| site 3 | 636 | 619 |
| site 4 | 565 | 539 |
| sevoflurane | ||
| site 1 | 587 | 572 |
| site 2 | 653 | 625 |
| site 3 | 678 | 660 |
| isoflurane | ||
| site 1 | 670 | 655 |
| site 2 | 642 | 622 |
| site 3 | 637 | 608 |
| site 4 | 573 | 552 |
| enflurane | ||
| site 1 | 657 | 638 |
| site 2 | 662 | 646 |
| site 3 | 625 | 596 |
| site 4 | 594 | 574 |
Of note is that none of the anaesthetics has proton affinities greater than water. An often stated phrase in PTR-MS research papers is that for a compound to be detected with any sensitivity it needs to have a proton affinity greater than that of water. This is not strictly true, because, and as mentioned earlier, proton transfer can be driven by the translational energy gained by the reagent ions in the electric field and through a reagent ion's internal energy, which may have been gained during its formation in the ion source or through collisional processes in the drift tube. Furthermore, the key quantity is not the difference in the proton affinities (ΔH), but changes in the free energy (ΔG), and proton transfer from protonated water to a volatile will be thermodynamically spontaneous if ΔG is negative. What is crucial is that once formed the protonated parent must spontaneously dissociate, otherwise it will react with the far more abundant water in the drift tube, dramatically reducing the sensitivity for detection of the parent molecule in PTR-MS analysis. Thus dissociation of a protonated parent, which can thermodynamically donate its proton to water, must occur on a timescale less than the average time for a collision between the protonated analyte and water in the drift tube. If this is not the case any protonated analyte will donate its proton to water in the drift tube with unit efficiency in a reverse reaction, and, given that water is in far higher concentrations than the analyte in the drift tube, the chemical equilibrium will be in favour of H3O+ production. Here the former is happening leading to copious amounts of product ions resulting from dissociative proton transfer, and this explains why they can be detected with high sensitivity with PTR-MS when using H3O+ at the reagent ion.
3.2.1. Desflurane
The four protonation sites are schematically represented below:
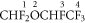
At 298 K proton transfer from H3O+ to desflurane is endothermic for all of the sites, with that to site 1 resulting in the lowest enthalpy (ΔH298 = +32 kJ mol−1) and free energy (ΔG298 = +15 kJ mol−1) changes.
Following proton transfer, the protonated desflurane fragments. The dominant fragment product ion observed in the experimental measurements, up to about a reduced electric field of 150 Td under 'normal' drift tube conditions and 170 Td under 'humid' drift tube conditions, is m/z 149.00 corresponding to loss of HF. This could occur from sites 1, 3, and 4, but loss from site 1 is the most likely as DFT investigations suggest that whilst loss from sites 3 and 4 is possible, further fragmentation is probable. Loss of HF does not involve a transition state, i.e. it is a barrierless process and this is so for loss of HF from the other three fluranes. The associated energetics are with ΔH298 = +81 kJ mol−1 and ΔG298 = +43 kJ mol−1, which are easily accessible in the drift tube.
3.2.2. Sevoflurane
The protonation sites for sevoflurane are defined below:
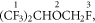
with protonation to site 3 being essentially thermoneutral at 298 K (ΔH298 = +6 kJ mol−1 and ΔG298 = −7 kJ mol−1). Given the small enthalpy and free energy changes, proton transfer from H3O+ to sevoflurane is likely to be efficient, meaning that the reaction rate coefficient must be at the collisional value. This agrees with the results obtained from a selected ion flow tube study of the reaction of H3O+ with sevoflurane [23], for which the experimentally determined room-temperature reaction rate coefficient of 3.4 × 10−9 cm3 s−1 is equal to the calculated collisional rate coefficient.
The dominant product ion is again formed by loss of HF from the protonated parent, and whilst loss from both sites 1 and 3 is possible with both leading to stable product ions, loss from site 3 is most likely and has the energetics ΔH298 = +50 kJ mol−1 and ΔG298 = +4 kJ mol−1.
3.2.3. Isoflurane
The four sites for protonation on the isoflurane molecule are schematically represented below:
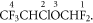
Protonation to site 1 is essentially thermoneutral at 298 K (ΔH298 = +14 kJ mol−1 and ΔG298 = −2 kJ mol−1). A SIFT study reports that, as found for sevoflurane, the reaction with H3O+ occurs at the collisional rate. The dominant product ion recommended for monitoring purposes is at m/z 67, CHFCl+, which can only occur via significant fragmentation. Extensive DFT investigations show that the only route to this ion is protonation at site 1 followed by the loss of HF. The resulting ion at m/z 165 has three possible further fragmentation routes but the one of interest here is fragmentation into CHFCl+ and CF3CHO. This fragmentation involves a Transition State and a migration of the Cl atom. The energetics for the initial loss of HF are ΔH298 = +54 kJ mol−1 and ΔG298 = +8 kJ mol−1. The further fragmentation and associated Transition State have the energetics ΔH298 = +174 kJ mol−1 and ΔG298 = +77 kJ mol−1 and ΔHǂ298 = +182 kJ mol−1 and ΔGǂ298 = +77 kJ mol−1, respectively.
3.2.4. Enflurane
The four sites for protonation on the enflurane molecule are defined as shown below:
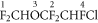
As mentioned earlier, the dominant product ion that can be used for exhaled breath monitoring purposes is at m/z 67, CHFCl+, as found for isoflurane. The most plausible fragmentation pathway to this ion is loss of HF following protonation at site 1 (ΔH298 = +27 kJ mol−1 and ΔG298 = +15 kJ mol−1) followed by further fragmentations to CHFO plus CF2 and CHFCl+, which appears to be barrierless. Whether this final fragmentation pathway is sequential or concerted is the subject of further theoretical investigations. The overall energetics are ΔH298 = +347 kJ mol−1 and ΔG298 = +203 kJ mol−1.
4. Discussion and concluding remarks
The calculated proton affinities of the anaesthetics are significantly less than any of the water clusters. This explains the low product ion signal intensities observed for all of the anaesthetics under the 'humid' drift tube conditions for low E/N (<100 Td) when the protonated water dimer is the dominant reagent ion species.
In the selected ion flow tube study of isoflurane and sevoflurane by Wang et al the reaction rate coefficients for their reactions with H3O+ were determined from the relative decay rates of H3O+ and  reagent ions, and the assumption that
reagent ions, and the assumption that  reacts at the collisional rate [22]. This assumption is based on their observation that all other
reacts at the collisional rate [22]. This assumption is based on their observation that all other  /ether reactions they had studied proceed at their collisional rates [30], although care has to be taken with this, because even highly exothermic charge transfer reactions may proceed with reaction rate coefficients substantially below the collisional value [31–33]. Nevertheless, Wang et al find that the relative decay rates of H3O+ and
/ether reactions they had studied proceed at their collisional rates [30], although care has to be taken with this, because even highly exothermic charge transfer reactions may proceed with reaction rate coefficients substantially below the collisional value [31–33]. Nevertheless, Wang et al find that the relative decay rates of H3O+ and  following their reaction with sevoflurane are in the ratio expected if both reactions proceed at their respective collisional rates. Wang et al also find that their experimentally determined reaction rate coefficient of 3.4 × 10−9 cm3 s−1 for the reaction of H3O+ with isoflurane is only slightly less than their calculated collisional rate value (kc = 3.5 × 10−9 cm3 s−1) implying, they claim, that the proton affinity of isoflurane must be slightly less than that of water. Although the measured reaction rate coefficient is within experimental error identical to the collisional value, the accuracy of the collisional rate coefficient is compromised because the polarizability and dipole moment of isoflurane had to be estimated. Nonetheless, the calculations presented in this paper confirm that their claim is correct for the reaction pathway leading to protonation on site 1 (ΔH298 = +14 kJ mol−1), with the reaction pathway being driven by entropic effects (ΔG298 = −2 kJ mol−1), and hence the reaction should proceed at the collisional rate, as experimentally observed.
following their reaction with sevoflurane are in the ratio expected if both reactions proceed at their respective collisional rates. Wang et al also find that their experimentally determined reaction rate coefficient of 3.4 × 10−9 cm3 s−1 for the reaction of H3O+ with isoflurane is only slightly less than their calculated collisional rate value (kc = 3.5 × 10−9 cm3 s−1) implying, they claim, that the proton affinity of isoflurane must be slightly less than that of water. Although the measured reaction rate coefficient is within experimental error identical to the collisional value, the accuracy of the collisional rate coefficient is compromised because the polarizability and dipole moment of isoflurane had to be estimated. Nonetheless, the calculations presented in this paper confirm that their claim is correct for the reaction pathway leading to protonation on site 1 (ΔH298 = +14 kJ mol−1), with the reaction pathway being driven by entropic effects (ΔG298 = −2 kJ mol−1), and hence the reaction should proceed at the collisional rate, as experimentally observed.
As expected from the calculated PAs and GBs of the four halogenated ethers, the intensities of the product ions (counts per second) are diminished at low reduced electric fields under both 'normal' and 'humid' tube conditions, but significantly much more under the 'humid' conditions. This is because proton transfer from the reagent ion H3O+.(H2O) to any of the anaesthetics is highly endothermic, and even translational energy gained in the electric field and increases in internal energies of the reagent ions through collisions with the buffer gas are unable to drive proton transfer. Therefore, only H3O+ has a significant role to play in the ionisation of the halogenated ethers in our PTR-MS investigations.
No protonated fluranes are observed, rather following proton transfer substantial fragmentation is observed through reaction pathways leading to product ions which often require substantial energy input. This in part may be coming from any internal energy that the H3O+ may contain when it is either generated in the ion source or through that gained in collisions with the buffer gas in the drift tube. Product ions also gain energy from the field, which leads to collisional dissociation. For the majority of product ion fragmentation channels, this energy input is substantial. We comment that extensive fragmentation is also reported in the selected ion flow tube mass spectrometric (SIFT-MS) study by Wang et al with the dominant product ion being m/z 117 (CF3CHCl+). This is difficult to explain given that the ion/molecule reaction processes in a SIFT are considered to be thermal [34].
Although substantial fragmentation is observed both in this PTR-MS and the SIFT-MS studies following proton transfer from H3O+ to isoflurane, and as expected more so in the PTR-MS study, there are major differences in the product ions. The SIFT study by Wang et al [22] reports a number of product ions that we have not observed, namely m/z 99 (assigned to be CF3CH2O+), m/z 119 (assigned to be CF3CFHO ), and m/z 147 (incorrectly assigned in the Wang et al paper to be CF2HOCHClCH+ which has a m/z value of 128 and is in any case a radical cation). The following product ions recorded in this PTR-MS study: m/z 51 (assigned to be
), and m/z 147 (incorrectly assigned in the Wang et al paper to be CF2HOCHClCH+ which has a m/z value of 128 and is in any case a radical cation). The following product ions recorded in this PTR-MS study: m/z 51 (assigned to be  ), m/z 115 (assigned to be C2H2F2ClO+), m/z 145 (assigned to be C3HF3ClO+), and m/z 163 (assigned to be C3F4ClO+) are not reported in the SIFT paper. The only common product ion recorded in both studies is at m/z 117 (C2HF3Cl+). An ion observed at m/z 67 in the SIFT study and assigned to be CF2HO+, is observed in our data, but because an ion signal is also observed in our mass spectrum at m/z 69 with an intensity to imply that that is associated with the 37Cl isotope (m/z 69 is not observed in the SIFT mass spectrum) we have assigned m/z 67 to be CHFCl+.
), m/z 115 (assigned to be C2H2F2ClO+), m/z 145 (assigned to be C3HF3ClO+), and m/z 163 (assigned to be C3F4ClO+) are not reported in the SIFT paper. The only common product ion recorded in both studies is at m/z 117 (C2HF3Cl+). An ion observed at m/z 67 in the SIFT study and assigned to be CF2HO+, is observed in our data, but because an ion signal is also observed in our mass spectrum at m/z 69 with an intensity to imply that that is associated with the 37Cl isotope (m/z 69 is not observed in the SIFT mass spectrum) we have assigned m/z 67 to be CHFCl+.
It is clear from this study, and others we have undertaken (for example see the paper by Malásková et al [26]), that a meaningful interpretation of the ion-chemistry occurring in the PTR-MS is challenging, and headway is only possible when the experimental observations are in combination with quantum mechanical computational calculations of the ion-molecule reaction pathways leading to the product ions.
Finally, we comment that the major objective of this study has been successfully accomplished, namely the identification of what product ion(s) should be monitored at a defined reduced electric field value for a given anaesthetic with high specificity under the conditions similar to those for breath samples for the PTR-TOF 8000 instrument we are using. In summary, these product ions and reduced electric fields to be used are as follow: desflurane, C3H2F5O+ (m/z 149.00), at an E/N of 120 Td; sevoflurane, C4H3F6O+ (m/z 181.01), at an E/N of 150 Td; and for both isoflurane and enflurane, CHFCl+ (m/z 66.98 and m/z 68.98), at an E/N of 200 Td. This provides us with the method to monitor the 'washout' characteristics of any one of the four inhalation anaesthetic present in a person following surgery, with the excretion from the body predominantly resulting via exhalation, because they are not efficiently metabolised. It is our intention that the washout measurements will be modelled using a simulation based on one developed for anaesthetics by King et al [35], and since breath concentrations of VOCs with low blood:air partition coefficients (λb:air ≤ 5, e.g. isoflurane has λb:air = 1.4 and sevoflurane has λb:air = 0.6) are very sensitive to breath flow  and blood flow
and blood flow  ideally to aid the modelling the measured breath concentrations (Cmeasured) of the anaesthetics should be normalised according to the Farhi equation
ideally to aid the modelling the measured breath concentrations (Cmeasured) of the anaesthetics should be normalised according to the Farhi equation  with no anaesthetic in the room air being breathed.
with no anaesthetic in the room air being breathed.
Acknowledgments
We thank the Marie Skłodowska-Curie Actions Innovative Training Network: Ion-Molecule Processes for Analytical Chemistry Technologies (IMPACT) (www.impact-h2020itn.com), which has supported this research through the European Commission's HORIZON 2020 Programme under Grant Agreement Number 674911. Ms Michaela Malásková and Mr David Olivenza-León are Early Stage Researchers attached to the IMPACT ITN. They contributed equally to the experimental investigations, and hence they should be considered as joint first authors. Dr Prema Chellayah acknowledges the financial support of the Ministry of Health Malaysia for her PhD programme in the Molecular Physics Group, School of Physics and Astronomy, at the University of Birmingham, UK.