Abstract
We report non-mass-dependent Fe isotopic data for troilite (FeS) inclusions from 10 iron meteorites, representing both non-magmatic (IAB) and magmatic groups (IIAB, IIIAB, IVA). No resolvable variations are present in the most neutron-rich isotope (58Fe), but small deficits (≈−0.1 ε) in 56Fe were observed in several inclusions. With the exception of several Ca–Al-rich inclusions in primitive meteorites, these are the first reported non-mass-dependent variations in Fe isotopes for material formed in the early solar system. Nucleosynthetic variations in Ni isotopes were previously reported in these same samples. The effects in Fe isotopes are not correlated with those in Ni, which suggests that the origins of the isotopic variations are decoupled from one another. The 56Fe deficits may represent incomplete mixing of the precursor dust in the protoplanetary disk. Alternatively, a parent body process (e.g., irradiation by galactic cosmic rays) may have modified the Fe isotopic compositions of some inclusions, which initially had homogeneous Fe isotopic compositions.
Export citation and abstract BibTeX RIS
1. Introduction
The gas and dust from which the solar system formed were derived from a variety of stellar sources and nucleosynthetic processes. This led to unique and distinct isotopic compositions of these dust grains. Models of protoplanetary disks suggest that mixing in the inner solar nebula was efficient and should have rapidly (≈1000 years) homogenized the starting materials such that any residual compositional heterogeneity on planetary scales is less than ≈10% (e.g., Boss 2008). Detection of non-mass-dependent nucleosynthetic isotopic variations among solar system objects can help to constrain the degree of homogenization of the material that formed the solar system and may also help to identify the stellar sources from which these materials originated. The degree of isotopic homogenization provides clues to the physical processes operating within the protoplanetary disk and yields important information regarding the distribution of short-lived radionuclides (e.g., 26Al, 60Fe) used to establish the chronology of the early solar system.
Nucleosynthetic isotopic variations in multiple elements have been reported in bulk meteorites (e.g., Dauphas et al. 2002; Andreasen & Sharma 2007; Trinquier et al. 2007, 2009; Leya et al. 2008; Qin et al. 2008; Chen et al. 2010; Moynier et al. 2012; Akram et al. 2013, 2015), indicating the preservation of some isotopic heterogeneity within the protoplanetary disk. Whether these variations represent incomplete mixing within the nebula or arise from later processing of initially homogenized starting materials remains an open question. In contrast to other Fe-group elements (e.g., Ti, Cr, Ni), analyses of whole rock meteorites indicate that Fe isotopes are homogenous among solar system bodies (Dauphas et al. 2004, 2008; Tang & Dauphas 2012). These three previous studies reported Fe isotopic data for a wide variety of meteorite classes, including the metal phase from iron meteorites. To date, non-mass-dependent variations in Fe isotopes have been reported only for several Ca–Al-rich inclusions (CAIs) from the Allende meteorite (Völkening & Papanastassiou 1989). High-precision measurements of non-mass-dependent effects in Fe isotopes for troilite (FeS) inclusions in iron meteorites are not yet available; however, several studies reported nucleosynthetic Ni isotopic variations in such samples (Quitté et al. 2006; Cook et al. 2008; Moynier et al. 2011). Quitté et al. (2006) reported Ni isotopic data defining a trend consistent with the preservation of an AGB component within the FeS. Cook et al. (2008) reported data more consistent with the admixture of Ni from a SN II. Chen et al. (2009) did not observe Ni isotopic anomalies in FeS inclusions from iron meteorites and challenged the previous findings. A later study by Moynier et al. (2011) reported results compatible with those of Cook et al. (2008).
In an attempt to better constrain the potential stellar source of the nucleosynthetic Ni isotopic variations in FeS inclusions from iron meteorites, Fe isotopes were analyzed in the same sample aliquots from 10 of the inclusions previously analyzed by Cook et al. (2008) for Ni isotopes. The measured Fe isotopic compositions were compared to potential compositions predicted from models of stellar nucleosynthesis that could explain the observed Ni variations. In addition to a stellar source(s) for the Fe isotopic variations, we consider effects operating on the meteorite parent bodies, such as galactic cosmic rays (GCRs) or the nuclear field shift (NFS), that may also perturb Fe isotopic ratios.
2. Experimental
2.1. Samples and Preparation
Ten FeS inclusions were analyzed from iron meteorites belonging to the non-magmatic group IAB and from magmatic groups IIAB, IIIAB, and IVA. These are aliquots of the same sample solutions previously analyzed for Ni isotopes (Cook et al. 2008), and the reader is referred to this earlier work for details of the sampling and digestion procedures. Three terrestrial Fe–Ni sulfides were also analyzed to verify that our chemical separation and mass spectrometry protocols are free from analytical artifacts. These samples include violarite (FeNi2S4), pentlandite ((Fe,Ni)9S8), and pyrrhotite ( S); the latter sample was chosen as an external standard. The terrestrial sulfides were digested in Teflon beakers at 120°C in a 2:1 mixture of concentrated HNO3 and HCl, following the procedure of Cook et al. (2008). In addition to the sulfide samples, two aliquots of the IRMM-014 Fe isotopic standard were processed through the Fe separation chemistry.
S); the latter sample was chosen as an external standard. The terrestrial sulfides were digested in Teflon beakers at 120°C in a 2:1 mixture of concentrated HNO3 and HCl, following the procedure of Cook et al. (2008). In addition to the sulfide samples, two aliquots of the IRMM-014 Fe isotopic standard were processed through the Fe separation chemistry.
2.2. Separation of Iron
Iron was separated from the matrix by adapting protocols reported by Dauphas et al. (2004), using a combination of anion and cation exchange chromatography in HCl media. An aliquot of the digested sample equivalent to ≈2000 μg Fe was dried in a Teflon beaker and re-dissolved in 0.2 ml 6M HCl. Sample solutions were loaded onto columns filled with 1 ml of anion exchange resin (AG1 × 8; 200–400 mesh) that had been cleaned and conditioned with 5 ml MQ, 5 ml 1M HNO3, 10 ml MQ, and 5 ml 6M HCl. After loading, the columns were washed with 6 ml 6M HCl, and Fe was eluted in 5 ml 0.4M HCl. Iron may not be completely separated from the matrix for samples with abundant transition metal contents using HCl media and anion resin (Schoenberg & von Blankenburg 2005); these elements commonly occur in FeS nodules in iron meteorites (Buchwald 1977). In particular, the separation of Cu and Zn from Fe may be problematic for sulfide samples using only anion resin (Dauphas et al. 2009). Therefore, the Fe fraction from the anion column was further purified by loading onto a column filled with 2 ml of cation exchange resin (AG50W × 8; 200–400 mesh) that had been cleaned and conditioned with 10 ml MQ, 10 ml 3M HCl, and 10 ml 10M HCl. The Fe fraction from the anion column was dried and re-dissolved in 0.1 ml 10M HCl for loading onto the cation column. After loading, the sample was allowed to equilibrate with the resin for 2 hr. Next, the column was washed with 4 ml 10M HCl, and Fe was eluted in 4 ml 3M HCl. The cation column provides a quantitative separation of Fe from all transition metals (cf. Saito 1984). Following the second column, the solutions were evaporated, and the Fe fractions were dried once in a 2:1 mixture of concentrated HNO3:HCl (0.45 ml) and once in concentrated HNO3 (0.45 ml). Lastly, all samples were taken up in ≈0.42M HNO3 for isotopic analysis. The total chemical yield for Fe was ≈100%. The full procedural blank was ≈0.040 μg Fe, which is insignificant compared to the amount of sample Fe (≈2000 μg) processed.
2.3. Mass Spectrometry
Isotopic measurements were made with a ThermoScientific Neptune Plus MC-ICPMS at the Institut für Geochemie und Petrologie (ETH Zürich), in medium-resolution mode, using standard sampler and skimmer Ni cones. Iron samples were introduced using the ThermoScientific Stable Introduction System (SIS), wet plasma, and an uptake rate of 100 μL/min. Iron analyses consisted of 15 measurements (20 integrations of 8.4 s) bracketed by measurements of the IRMM-014 isotopic standard. All four Fe isotopes (54Fe, 56Fe, 57Fe, and 58Fe) were measured simultaneously in static mode along with 53Cr and 60Ni, which were used to correct interferences on 54Fe from 54Cr and on 58Fe from 58Ni. The 56Fe signal was measured using a 1010 Ohm resistor, and the 53Cr and 60Ni signals were measured using 1012 Ohm resistors. Sample solutions of 10 ppm were analyzed, which provided beams of ≈100 V on the most abundant isotope (i.e., 56Fe). A single analysis generally consumed ≈65 μg of Fe. A washout time of 210 s was used after the analysis of a sample or standard solution. Each analytical session was preceded by a single 300 s measurement of the electronic baseline, which was subtracted from all signal intensities. Instrumental mass bias was corrected using the exponential law with 57Fe/54Fe = 0.36255 (Taylor et al. 1992). Iron isotopic ratios (56Fe/54Fe and 58Fe/54Fe) are reported as the parts per 10,000 deviation relative to the bracketing standard, IRMM-014, using the following notation: εiFe, where i = 56 or 58. Uncertainties reported for individual samples (Tables 1 and 2) represent 95% confidence intervals based on the 15 repeat measurements of each solution. They were calculated using the following:
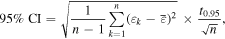
where  is the mean value of the 15 repeats and
is the mean value of the 15 repeats and  is Student's t-value for a two-sided 95% confidence interval with
is Student's t-value for a two-sided 95% confidence interval with  degrees of freedom (e.g., Qin et al. 2008).
degrees of freedom (e.g., Qin et al. 2008).
Table 1. Iron Isotopic Compositions of Terrestrial Iron
| Sample | ε56Fe ± 95CIa | ε58Fe ± 95CI |
|---|---|---|
| pyrrhotite | ||
| #1 | −0.02 ± 0.05 | −0.12 ± 0.27 |
| #2 | −0.01 ± 0.05 | 0.14 ± 0.16 |
| #3 | −0.05 ± 0.03 | −0.06 ± 0.20 |
| #4 | −0.02 ± 0.04 | 0.06 ± 0.12 |
| #5 | 0.03 ± 0.05 | 0.18 ± 0.18 |
| #6 | −0.06 ± 0.03 | 0.03 ± 0.15 |
| #7 | −0.03 ± 0.03 | −0.02 ± 0.16 |
| #8 | −0.04 ± 0.02 | −0.15 ± 0.20 |
| #9 | 0.01 ± 0.05 | 0.12 ± 0.23 |
| #10 | −0.05 ± 0.03 | −0.07 ± 0.16 |
| #11 | −0.01 ± 0.04 | 0.03 ± 0.18 |
| #12 | 0.01 ± 0.03 | 0.06 ± 0.13 |
| #13 | 0.00 ± 0.03 | 0.06 ± 0.14 |
| #14 | −0.03 ± 0.05 | 0.00 ± 0.12 |
| mean ± 2SD | −0.02 ± 0.05b | 0.02 ± 0.19 |
| violarite | −0.03 ± 0.03 | 0.10 ± 0.20 |
| pentlandite | −0.06 ± 0.02 | −0.03 ± 0.19 |
| IRMM-1 | 0.02 ± 0.04 | −0.13 ± 0.20 |
| IRMM-2 | 0.02 ± 0.04 | −0.17 ± 0.15 |
Note.
a95CI is the 95% confidence interval based on 15 repeat measurements of each sample. bValues are in bold in order to highlight the mean values for the pyrrhotite from the other individual measurements.Download table as: ASCIITypeset image
Table 2. Iron Isotopic Compositions of Troilite Inclusions from Iron Meteorites
| Sample | Group | ε56Fe ± 95CIa | ε58Fe ± 95CI |
|---|---|---|---|
| Canyon Diablo | IAB | −0.06 ± 0.03 | 0.05 ± 0.17 |
| Toluca | IAB | −0.02 ± 0.05 | 0.18 ± 0.13 |
| Mundrabilla | IAB | −0.02 ± 0.03 | −0.07 ± 0.15 |
| Odessa | IAB | −0.03 ± 0.02 | 0.04 ± 0.16 |
| Gressk | IIAB | −0.10 ± 0.02 | −0.09 ± 0.18 |
| Augustinovka | IIIAB | −0.07 ± 0.04 | 0.04 ± 0.16 |
| Bella Roca | IIIAB | −0.07 ± 0.04 | 0.04 ± 0.15 |
| Chupaderos | IIIAB | −0.04 ± 0.02 | −0.11 ± 0.07 |
| Grant | IIIAB | −0.08 ± 0.04 | 0.03 ± 0.15 |
| Gibeon | IVA | −0.12 ± 0.03 | −0.02 ± 0.12 |
Note.
a95CI is the 95% confidence interval based on 15 repeat measurements of each sample.Download table as: ASCIITypeset image
3. Results
3.1. Terrestrial Samples
Over the duration of the study, 14 aliquots of our terrestrial pyrrhotite were processed through the full chemical procedure described in Section 2.2. The isotopic measurements of these replicates define an external reproducibility (2SD) for ε56Fe and ε58Fe of ±0.05 and ±0.19, respectively (Table 1). This level of analytical resolution represents an improvement compared to previous studies (e.g., Dauphas et al. 2004; Tang & Dauphas 2012). The εiFe values measured on the two aliquots of IRMM-014 processed through ion exchange chemistry show no resolvable effects, demonstrating that the analytical procedure is free from artifacts. After chemical separation of Fe, all terrestrial sulfides had Ni/Fe ≤ 2.2 × 10−5 and Cr/Fe ≤ 6.1 × 10−7. These ratios are far below the thresholds required to make accurate measurements of Fe isotopes in the presence of isobaric interferences from Cr and Ni (Dauphas et al. 2009). The above data demonstrate the effectiveness of our chemical separation protocol and the accuracy of the overall method.
3.2. Troilite Inclusions from Iron Meteorites
All FeS inclusions (Table 2) display ε58Fe values that are identical to the terrestrial isotopic standard within the overall analytical resolution of ±0.19. In contrast, six of the inclusions show small but clearly resolved deficits in ε56Fe (i.e., their ε56Fe values differ from the isotopic standard by > ± 0.05). These deficits occur in each of the iron meteorite groups sampled. Like the terrestrial samples, the chemically purified FeS samples have insignificant levels of isobaric interferences (Ni/Fe ≤ 1.4 × 10−5 and Cr/Fe ≤ 6.9 × 10−7), which cannot account for the observed ε56Fe deficits. We note that it is not currently possible to conclusively identify which Fe isotope is anomalous. One possibility is that the ε56Fe values for FeS samples reflect a true variation in 56Fe. However, an excess of either 54Fe or 57Fe, used to correct the instrumental mass bias, can also lead to negative ε56Fe values. Thus, we restrict our discussion to ε56Fe values internally normalized to 57Fe/54Fe and do not discuss absolute 56Fe abundances.
4. Discussion
4.1. Possible Stellar Source of Nucleosynthetic Nickel Isotopic Variations
Before discussing the results for the Fe isotopes in detail, we first present a re-evaluation of the stellar component responsible for the observed nucleosynthetic Ni isotopic variations (Cook et al. 2008) in the FeS inclusions. These results are then used to explore the potential causes for the observed Fe isotopic variations. Cook et al. (2008) presented trends for isotopic mixtures between terrestrial Ni and that produced in three stellar environments, as well as for potential spallation effects on FeS from GCRs. In the current study, we take a similar approach. In order to constrain the possible stellar source that produced the component responsible for the observed εiNi variations, isotopic mixtures were calculated between terrestrial Ni (Gramlich et al. 1989) and the yields from various nucleosynthetic stellar models, including type Ia supernovae (SN Ia) (Iwamoto et al. 1999; Travaglio et al. 2004; Maeda et al. 2010; Travaglio et al. 2011; Seitenzahl et al. 2013), type Ib,c supernovae (SN Ib,c) (Woosley et al. 1995), a core collapse type II supernova (SN II) for a 25M⊙ progenitor (Rauscher et al. 2002), and asymptotic giant branch (AGB) stars (Domínguez et al. 2011; Pignatari et al. 2014). For the SN II mixtures, the yields from each of the eight individual nuclear burning zones within the pre-supernova star, as well as the yields for the bulk ejecta from the explosion (Rauscher et al. 2002), were considered. In all, 65 stellar models were considered, and mixing lines between each stellar source and terrestrial Ni were calculated.
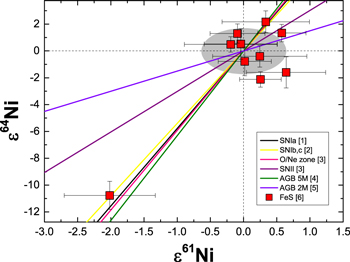
Figure 1 shows selected trends expected for mixtures between terrestrial Ni and individual stellar models. Eight mixtures provide isotopic compositions that are within uncertainty of the most extreme observed variations (i.e., Mundrabilla). For visual clarity, only four of these are shown: SN Ia, SN Ib,c, SN II (O/Ne zone), and AGB (5M⊙ Z = 0.02). These represent the mixtures that provide the closest match from each of the four types of stellar environments (SN Ia, SN Ib,c, SN II, and AGB). Three additional SN Ib,c mixtures and one AGB (5M⊙ Z = 0.01) mixture give similar results to those shown in Figure 1. These trends confirm that the Ni isotopic variations in FeS are consistent with a nucleosynthetic origin and that a variety of stellar environments could represent the source composition needed to explain the observations.
Figure 1. Selected isotopic mixing lines between terrestrial Ni and that from individual stellar yields, along with the measured Ni isotopic compositions of meteoritic FeS inclusions. Four trends represent the mixtures from each of the four types of stellar environments (SN Ia, SN Ib,c, SN II [O/Ne zone], and AGB) that provide the closest match to the largest observed variations (cf. Figure 2 in Cook et al. 2008). The two non-matching trends represent mixtures favored in earlier studies: Quitté et al. 2006 (AGB 2M⊙) and Cook et al. 2008 (SN II; 15M⊙ progenitor). The gray area represents the analytical resolution in ε61Ni–ε64Ni space. Literature data for stellar models: (1) Maeda et al. (2010), (2) Woosley et al. (1995), (3) Rauscher et al. (2002), (4) Pignatari et al. (2014), (5) Domínguez et al. (2011). FeS data are from (6) Cook et al. (2008).
Download figure:
Standard image High-resolution image4.2. Iron Isotopic Variations: Nucleosynthetic Predictions versus Observations
Previous studies of iron isotopes from a wide variety of meteorite types (Dauphas et al. 2004, 2008; Tang & Dauphas 2012) did not observe any resolvable variations and concluded that Fe isotopes were homogeneously distributed in the protoplanetary disk. These previous studies placed a particular emphasis on the homogeneity of ε58Fe because the low abundance of 58Fe (0.28%; Taylor et al. 1992) makes it a sensitive proxy for mixing between sources with disparate Fe isotopic compositions; in general, our calculations reveal that mixtures containing a small (≈0.0001%) amount of stellar Fe from any of the 65 sources considered would have resolvable ε58Fe values relative to pure terrestrial Fe. The lack of resolved ε58Fe variations in FeS is consistent with previous studies of bulk meteorites, and this ratio by itself supports a homogeneous distribution of Fe isotopes within the early nebula.
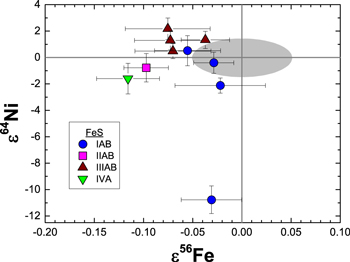
In contrast to ε58Fe, six FeS inclusions (Table 2) show small but resolvable deficits in ε56Fe. These are the first reported non-mass-dependent Fe isotopic variations for samples other than CAIs (Völkening & Papanastassiou 1989). Unlike Ni isotopes in the same samples, which show excesses and deficits in ε64Ni, only deficits are observed in ε56Fe (Figure 2). This raises the question of whether additional constraints on the possible stellar source of the Ni isotopic variations could be provided by comparing the measured Fe isotopic compositions to collateral effects on Fe isotopes predicted by the mixtures that match the Ni isotopic data. For this comparison, the most anomalous Ni isotopic composition (i.e., Mundrabilla) was chosen. First, mixtures were calculated that reproduced the magnitude of the observed ε64Ni deficit in Mundrabilla. Next, the expected collateral effects on Fe isotopes were calculated. A dilution factor was included that accounted both for the Fe/Ni ratio of the nucleosynthetic material for the relevant stellar model and the Fe/Ni ratio for average solar system material (Lodders 2003). The predicted collateral effects on Fe isotopes in Mundrabilla FeS are large (Figure 3) and would be well-resolved from the terrestrial reference standard with our current analytical precision; they are clearly inconsistent with the measured values. Similarly, matching the Fe isotopic composition of FeS from Gibeon to mixtures of stellar and terrestrial Fe predicts collateral effects on Ni isotopes that do not equal those observed. The absence of the predicted effects, in combination with the lack of correlation (r2 = 0.09) between ε56Fe and ε64Ni (Figure 2), indicate that the Ni and Fe isotopic anomalies are decoupled. Thus, the Fe isotopes do not help to constrain the possible stellar source responsible for the nucleosynthetic Ni variations.
Figure 2. Measured values for ε56Fe (this study) and ε64Ni (Cook et al. 2008) for FeS inclusions in iron meteorites. The gray area represents the analytical resolution in ε56Fe–ε64Ni space.
Download figure:
Standard image High-resolution imageFigure 3. Measured ε56Fe and ε58Fe values in FeS from the IAB iron Mundrabilla, along with the predicted collateral effects on those ratios for isotopic mixtures between terrestrial Fe and the four stellar sources that produce a match to the Ni isotopic variations (see Figure 1), as well as the stellar source suggested in Cook et al. (2008). Note that the SN Ia mixture predicts values that plot off-scale and is not shown. References for stellar models are the same as in Figure 1. The gray area represents the analytical resolution in ε56Fe–ε58Fe space.
Download figure:
Standard image High-resolution imageDecoupling of nucleosynthetic variations have been reported in several studies. For example, Wang et al. (2011) reported an absence of Fe isotopic variations in acid leachates prepared from whole rock chondrites that contained large nucleosynthetic Cr isotopic variations. Similarly, Fehr et al. (2006) did not observe Te nucleosynthetic isotopic anomalies in bulk carbonaceous chondrite leachates for which large variations in Zr isotopes were reported (Schönbächler et al. 2005). In addition, Walker (2012) also showed that nucleosynthetic variations are absent in Os isotopes in iron meteorites that are anomalous for isotopes of other siderophile elements like Ru (Chen et al. 2010) and Mo (Burkhardt et al. 2011). One possible explanation for the lack of correlation between nucleosynthetic effects on Ni and Fe isotopes is that Ni and Fe resided in different presolar carriers. Condensation calculations for products from a SN II (Fedkin et al. 2010) showed that the redox environment can affect the nature of the host phase(s) for Fe and Ni. In oxidizing environments (e.g., O/Ne zone), Ni is predicted to be hosted in Fe-poor metal, whereas Fe will primarily condense into silicates and oxides. Thus, fractionation of Fe from Ni may occur during the condensation of some stellar products and could provide a mechanism to separate these two elements into different carrier phases. Subsequent incorporation of varying amounts of each carrier (i.e., metal versus silicate) into the iron meteorite parent bodies could lead to a decoupling of the nucleosynthetic signal. Alternatively, thermal processing and preferential destruction of certain carriers within the protoplanetary disk could have led to reservoirs with different isotopic compositions for one element but not another (e.g., Trinquier et al. 2009; Larsen et al. 2011; Walker 2012; Akram et al. 2015).
4.3. Neutron Capture Effects
An alternative possibility is that the ε56Fe deficits do not represent isotopic variability in the nebula, but rather are the result of processes acting on the meteorite parent bodies, which began with homogeneous Fe isotopic compositions. Such an explanation may account for the seemingly contradictory occurrence of ε56Fe deficits accompanied by homogeneous ε58Fe values. For example, while in outer space, meteoroids are bombarded by high-energy GCRs. This interaction can induce nuclear reactions within the meteoroid, leading to changes in its isotopic composition; such effects are well-documented in meteorites (e.g., Masarik & Reedy 1994; Masarik 1997; Leya et al. 2003; Leya & Masarik 2013). Primary spallation reactions by GCR lead to the production of secondary neutrons; these neutrons may then be captured by some nuclei if they are slowed down sufficiently by collisions as they travel through the target. The modification of isotopic compositions due to neutron capture reactions have been reported in iron meteorites for multiple elements, including Ag (Matthes et al. 2015), Ru (Fischer-Gödde et al. 2015), Os (e.g., Walker 2012; Wittig et al. 2013), Pt (e.g., Kruijer et al. 2013), and W isotopes (e.g., Markowski et al. 2006; Kruijer et al. 2013). Furthermore, GCR effects are not uniform because their magnitude changes as a function of depth below the surface (e.g., Masarik 1997; Leya & Masarik 2013). Thus, this depth dependency can lead to isotopic differences among irons within a particular group and also between different pieces of the same meteorite (e.g., Walker 2012; Kruijer et al. 2013; Qin et al. 2015).
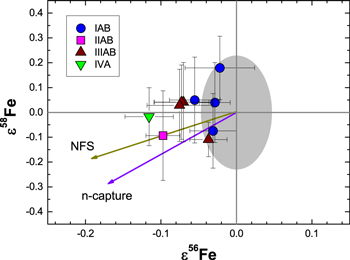
To assess potential neutron capture effects on Fe isotopes, the neutron capture resonance integrals for Fe isotopes from Mughabghab (2006) were used to calculate the possible influence of GCRs on ε56Fe and ε58Fe. Neutron capture effects shift ε56Fe and ε58Fe to lower values (Figure 4). The magnitude of the effect on ε58Fe is larger than on ε56Fe (ε58Fe/ε56Fe = 1.68). However, for the small ε56Fe deficits observed (≥−0.12), the corresponding effects on ε58Fe are nearly equal to or less than our analytical resolution (±0.19), and the measured ε58Fe values agree with this expectation. A GCR origin for the ε56Fe deficits can also explain the variability among members of the same group (e.g., IIIAB), which experienced different degrees of irradiation due to origins at varying depths within the parent body. Overall, the predicted and measured values are consistent, which supports the potential modification of an originally homogeneous Fe isotopic composition due to GCR effects. Future studies that combine measurements of Fe isotopes with those of Ti and/or Cr may help to identify GCR effects in FeS inclusions from iron meteorites. Neutron capture effects have been shown to occur on Ti isotopes for lunar and meteoritic samples (Zhang et al. 2012), and significant GCR effects on Cr isotopes are predicted for samples with long exposure ages and elevated Fe/Cr ratios (Leya et al. 2003).
Figure 4. Measured ε56Fe and ε58Fe values for FeS inclusions in iron meteorites, along with the predicted trends for GCR-induced neutron capture reactions (n-capture) and nuclear field shift (NFS) effects. The gray area represents the analytical resolution in ε56Fe–ε58Fe space.
Download figure:
Standard image High-resolution image4.4. Nuclear Field Shift Effects
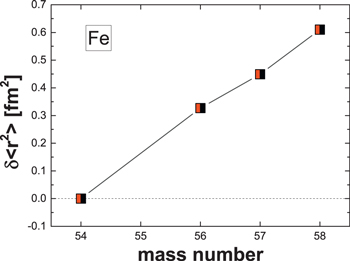
Fujii et al. (2006a) suggested that effects due to the NFS could provide an alternative explanation for isotopic variations in CAIs that had initially been attributed to a nucleosynthetic origin. Hence, it is useful to consider NFS effects when non-mass-dependent isotopic variations are observed. The NFS effect on isotope fractionation was described by Bigeleisen (1996) and Schauble (2007). The NFS is expressed during chemical exchange reactions, and it causes isotopic variations that deviate from a mass-dependent fractionation trend. These effects arise from differences in the sizes and shapes of nuclei among isotopes of a given element, which are in turn due to the different numbers of neutrons within the nucleus. In general, NFS effects are largest for isotopes of heavy elements (e.g., U), and they decrease with atomic mass. The effects are predicted to be small for light elements (A < ≈ 100). Because NFS effects are proportional to the nuclear charge radii, they do not follow a linear dependence on atomic mass. This nonlinear behavior is evident in diagrams of nuclear charge radii versus atomic mass (e.g., Aufmuth et al. 1987; Fujii et al. 2009) and is known as odd–even staggering. Figure 5 shows such a diagram for Fe isotopes. The nuclide 57Fe, the only Fe isotope with an odd number of neutrons, plots slightly off the linear trend between its even-number neutron neighbors, which suggests it may be prone to NFS effects.
Figure 5. Variation in the mean-squared nuclear charge radii,  , plotted against mass number for Fe isotopes. Nuclear radii data are from Angeli (2004).
, plotted against mass number for Fe isotopes. Nuclear radii data are from Angeli (2004).
Download figure:
Standard image High-resolution imageNuclear fields shift effects are particularly important during chemical reactions that involve a change in redox state. For redox reactions that involve the transfer of s-orbital electrons, the heavy isotopes will be preferentially concentrated in the more oxidized phase (Bigeleisen 1996; Schauble 2007). Qualitatively, NFS effects predict that the heavy Fe isotopes should show an enhanced preference for the oxidized phase (FeS) over the reduced phase (metal) in iron meteorites because the redox reaction (Fe0 to Fe2+) involves a change in s-orbital electrons. A slight excess of 57Fe in the FeS would lead to apparent deficits in ε56Fe and ε58Fe because 57Fe is used in the ratio to correct the instrumental mass bias. Experimental work by Fujii et al. (2006b) did not find conclusive evidence for NFS effects on Fe isotopes, but some results show slight departures from the trend expected for a purely mass-dependent fractionation. Additionally, theoretical predictions for the maximum NFS effects in Fe-group elements of around 0.2–0.3ε per amu (Moynier et al. 2013) are comparable to the observed ε56Fe deficits in FeS.
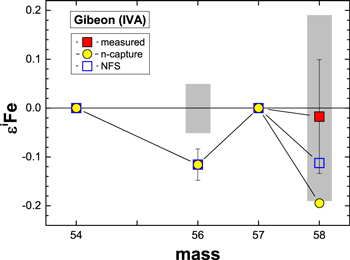
Values for nuclear charge radii (Angeli 2004) were used in conjunction with Equation (1) from Fujii et al. (2006a) to calculate NFS effects on Fe isotopic ratios. The expected trend (ε58Fe/ε56Fe = 0.97) is distinct from that for neutron capture effects (Figure 4), but like those for neutron capture effects, the predicted shifts in ε58Fe are too small to resolve. The NFS trend provides a slightly better fit to the data, but a lack of correlated effects (r2 = 0.08) in ε56Fe and ε58Fe preclude differentiating between the two trends. The FeS inclusion from Gibeon displays the largest ε56Fe deficit. Comparing the effects from the NFS to GCR-induced effects (Figure 6) shows a somewhat better overall match between the NFS and the measured values. However, the effects from neutron capture reactions are nearly at the limit of our ability to resolve a difference in ε58Fe from the terrestrial standard, so GCR effects cannot be ruled out.
Figure 6. The εiFe values for an FeS inclusion from the Gibeon iron meteorite, which has the largest observed ε56Fe deficit. Also shown are the corresponding ε58Fe deficits expected for GCR-induced neutron capture reactions (n-capture) or nuclear field shift (NFS) effects that reproduce the ε56Fe deficit. The gray boxes represent the analytical resolution of the measurements.
Download figure:
Standard image High-resolution image5. Conclusions
We report the first non-mass-dependent deviations from the terrestrial standard for Fe isotopes in iron meteorites. They are present as small ε56Fe deficits in some FeS inclusions. Conversely, ε58Fe values show no resolvable variations. Determining the level of Fe isotopic homogeneity within the early nebula is important because of the implications for the 60Fe–60Ni chronometer, which is based on the decay of the short-lived nuclide 60Fe ( Ma; Rugel et al. 2009). A non-uniform distribution of Fe isotopes in the protoplanetary disk would complicate, or perhaps prevent, the use of 60Fe as a tool to determine the timing of events in the early solar system. If the distribution of 60Fe was homogenized to the same level as observed for ε58Fe in FeS (i.e., ±17 ppm; 2SD), this would correspond to apparent shifts in 60Fe–60Ni ages of only ±64 years; thus, such a level of variability in 60Fe would have no detectable effect on ages based upon 60Fe–60Ni systematics.
Ma; Rugel et al. 2009). A non-uniform distribution of Fe isotopes in the protoplanetary disk would complicate, or perhaps prevent, the use of 60Fe as a tool to determine the timing of events in the early solar system. If the distribution of 60Fe was homogenized to the same level as observed for ε58Fe in FeS (i.e., ±17 ppm; 2SD), this would correspond to apparent shifts in 60Fe–60Ni ages of only ±64 years; thus, such a level of variability in 60Fe would have no detectable effect on ages based upon 60Fe–60Ni systematics.
Mixtures of terrestrial material with the stellar yields predicted for several different stellar sources provide a potential match to the observed Ni isotopic data previously reported in these samples (Cook et al. 2008), but the predicted collateral effects on Fe isotopes are absent. If the variations in Ni and Fe isotopes represent a nucleosynthetic origin, the decoupling of these effects suggests that these two elements resided in different presolar carriers; processing and/or variable incorporation of these carriers into the meteorite parent bodies could then account for the observed εiNi and εiFe values. However, the homogenous ε58Fe observed in this study and previous ones (e.g., Tang & Dauphas 2012) seems at odds with a scenario in which the small ε56Fe deficits observed in some FeS inclusions represent a residual heterogeneity in the nebular material from which the solar system was made.
Processes acting on the iron meteorite parent bodies may have modified Fe isotopes with an initially homogeneous composition; such processes provide likely alternatives to nebular heterogeneity as the origin of the observed ε56Fe deficits. One potential process is neutron capture reactions induced by GCR bombardment of the sample while in outer space. Another process may have been NFS effects induced by redox reactions during separation of Fe–FeS melts. Both processes cause non-mass-dependent isotopic fractionations and can produce ε56Fe deficits. At the current analytical resolution, it is not possible to differentiate between these two processes. However, this may be possible in the future if larger deficits are observed that show a clear correlation between ε56Fe and ε58Fe. Such observations would also help to determine whether the ε56Fe deficits in FeS samples originated on meteorite parent bodies or were inherited from materials in the protoplanetary disk.
We thank Brad Meyer, Waheed Akram, and Ingo Leya for helpful discussions. Two anonymous reviewers provided comments that lead to improvements. This work was carried out in the framework of the National Center for Competence in Research "PlanetS" supported by the Swiss National Science Foundation (SNSF). Support was also provided by the European Research Council under the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC Grant agreement n° [279779]. The lead author dedicates this manuscript to Patricia A. Lawrence, a.k.a. Dr. Mom, who made everything possible.