ABSTRACT
The gas-phase optical absorption spectrum of the thiophenoxy radical (C6H5S), a diffuse interstellar band (DIB) candidate molecule, was observed in the discharge of thiophenol using a cavity ringdown spectrometer. The ground-state rotational constants of the thiophenoxy radical were theoretically calculated, and the excited-state rotational constants were determined from the observed rotational profile. The rotational profile of a near prolate molecule having C2v symmetry was simulated on the basis of a rotational distribution model by radiation and collisions. Although the simulated profile did not agree with the observed DIBs, the upper limit of the column density for the thiophenoxy radical in the diffuse clouds toward HD 204827 was evaluated to be 2 × 1013 cm−2. The profile simulation indicates that rotational distribution by radiation and collisions is important to reproduce a rotational profile for a DIB candidate and that the near prolate C2v molecule is a possible candidate for DIB with a band width variation dependent on the line of sight.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
Although several hundreds of diffuse interstellar bands (DIBs) have been detected in visible and near infrared regions, DIBs remain one of the longest standing unsolved problems in spectroscopy and astrochemistry (Heger 1922; Hobbs et al. 2008, 2009; Dahlstrom et al. 2013). One of the best approaches to identify DIB carrier material is the generation and measurement of DIB candidate molecules in the laboratory to compare their absorption spectra with astronomically observed DIB spectra.
Polyaromatic hydrocarbons in the gas phase are potential DIB candidate molecules, and sulfur-containing molecules account for 10% of detected interstellar molecules. Thus, a sulfur-substituted benzene derivative is a good DIB candidate. Although it is difficult for a stable benzene derivative to have an optical transition, such transition is possible for a radical species.
Shibuya et al. (1988) reported the origin band of an electronic transition of the thiophenoxy radical (C6H5S) at 5172 ± 1 Å in air. The band is close to the 5170 Å DIB detected toward HD 204827 (Hobbs et al. 2008). To compare the DIB and the band of the thiophenoxy radical, an accurate transition wavelength is necessary, and a rotational profile based on rotational distribution in diffuse clouds should be estimated.
The rotational distribution is determined as a result of radiation and collision in diffuse clouds. Oka et al. (2013) have developed a model of rotational distribution by radiation and collisions for a linear molecule. For a near prolate molecule with C2v symmetry, Ka rotation around the a-axis is influenced by collision alone and Kc rotation around the c-axis is influenced by both radiation and collision, because a permanent dipole moment exists along the a-axis. This difference results in a special rotational distribution when the radiative temperature Tr and the kinetic temperature Tk are not in equilibrium. Generally, the temperature condition of a diffuse cloud is Tr < Tk. The special rotational distribution is necessary to simulate a rotational profile of a DIB. In this study, we simulate the rotational profile of the thiophenoxy radical using the rotational distribution by radiation and collisions.
In addition to the rotational profile, an accurate transition wavelength of the electronic transition of the thiophenoxy radical is essential to compare the band with DIBs. Recently, to detect a high-resolution spectrum in the laboratory, we have developed a cavity ringdown (CRD) spectrometer in which a discharge cell is installed. In this paper, we report the high-resolution spectrum and the estimated rotational profile as a DIB for the thiophenoxy radical, and we consider whether this radical is a potential DIB carrier.
2. EXPERIMENTAL DETAILS
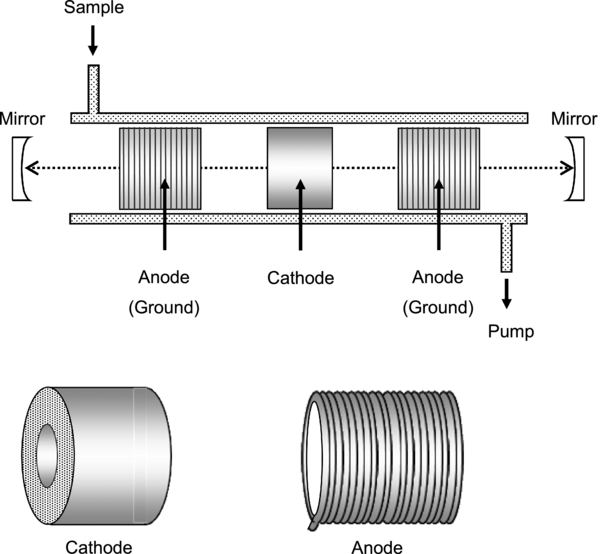
The CRD spectrometer was developed to observe the optical absorption spectra of DIB candidate molecules in the laboratory. The CRD spectrometer consists of a tunable pulse laser system, an optical cavity, and a discharge device. The tunable pulse laser beam is taken from a dye laser (ND6000, Continuum) with a resolution of 0.1 cm−1 pumped by a Nd:YAG laser (355 nm, Surelit). The optical cavity is constructed with two high-reflectivity mirrors (R > 99.995%, Los Gates Research). The hollow cathode glow discharge cell illustrated in Figure 1 is evacuated by a rotary pump. The discharge is produced by a pulsed voltage with a pulse width of 1 ms. Typically, a sample gas without a buffer gas is used in the discharge system. The discharge pulse is synchronized with the laser pulse by a custom-built pulse generator. The entire experiment runs at 10 Hz, and the ringdown signal is displayed on an oscilloscope. The signal is transferred to a data acquisition system including a ringdown calculation function that was developed with LabVIEW.
Figure 1. Schematic of the discharge cell and electrodes of the cavity ringdown spectrometer. The distance between the two mirrors is 75 cm. The discharge cell (internal diameter 3.3 cm) was made from Pyrex glass. The cathode (3 cm long, internal diameter 1 cm) was made of stainless steel. The anode (3 cm diameter) was made from a copper coil.
Download figure:
Standard image High-resolution imageThe thiophenoxy radical was produced by a discharge of 1000 V with a sample gas of thiophenol (C6H5SH, 0.25 torr). The C2 and Ar spectra were used to calibrate the wavelength of a thiophenoxy radical spectrum.
3. RESULTS AND DISCUSSION
3.1. Absorption Spectrum of the Thiophenoxy Radical
Shibuya et al. (1988) studied the thiophenoxy radical in the gas phase by laser-induced fluorescence and reported bands between 5200 and 4932 Å. The observed bands were presumed to be the 2A2 ← 2B2 electronic transition based on the theoretical calculation of the phenoxy radical (C6H5O) reported by Chang et al. (1975). However, Lim et al. (2006) reported the ground state of the thiophenoxy radical as 2B1. We calculated the energy levels of the first (2B2), second (2A2), third (2B1), fourth (2A1), and fifth (2B1) excited states of the thiophenoxy radical to be 0.43, 2.46, 3.01, 3.95, and 4.22 eV, respectively, using the theoretical calculation of TD-B3LYP/cc-pVTZ by Gaussian 03 (Frisch et al. 2003). The first excited state is optically forbidden from the ground state, and the transitions to the second and third excited states are b-type and a-type, respectively. Shibuya et al. (1988) reported origin and vibronic bands at 5172 and 5046 Å in air, respectively. Although a stellar line at the wavelength of the vibronic band is an interference factor, the origin band is suitable for comparison with a DIB. We observed the absorption spectrum of the origin band using the CRD spectrometer, as shown in Figure 2. The rotational profile of the band was of b-type; thus, the present band can be assigned to the 2A2 – X2B1 electronic transition.
Figure 2. Observed absorption spectrum of the 2A2–X2B1 transition of the thiophenoxy radical using the cavity ringdown spectrometer and the simulated rotational profile at 300 K by PGOPHER.
Download figure:
Standard image High-resolution imageTo analyze the rotational profile, we employed the ground-state rotational constants obtained by B3LYP/cc-pVTZ and assumed a rotational temperature of 300 K in the discharge because the rotational temperature of C2 was measured to be 300 K in the same hollow cathode glow discharge cell. When these constants and temperature are fixed, the observed rotational profile gives the constants of ΔA and Δ (
( = (B + C)/2), which correspond to the excited-state rotational constants as differences from the ground-state constants. However, simultaneous determination of ΔA and Δ
= (B + C)/2), which correspond to the excited-state rotational constants as differences from the ground-state constants. However, simultaneous determination of ΔA and Δ could not be converged in contour fitting with PGOPHER (version 8.0, Western). To solve this difficulty in fitting we calculated the excited-state rotational constants by B3LYP/cc-pVTZ and obtained the calculated values of ΔA and Δ
could not be converged in contour fitting with PGOPHER (version 8.0, Western). To solve this difficulty in fitting we calculated the excited-state rotational constants by B3LYP/cc-pVTZ and obtained the calculated values of ΔA and Δ . Using the fixed ratio of ΔA to Δ
. Using the fixed ratio of ΔA to Δ , i.e.,
, i.e.,  , we determined ΔA, Δ
, we determined ΔA, Δ , and the transition frequency T00 by manual manipulation of the constants by comparing the simulated and observed spectra. The obtained constants are listed in Table 1, and the profile comparison is shown in Figure 2.
, and the transition frequency T00 by manual manipulation of the constants by comparing the simulated and observed spectra. The obtained constants are listed in Table 1, and the profile comparison is shown in Figure 2.
Table 1. Obtained Molecular Constants of C6H5S in cm−1
| State | Ground a | Excited b |
|---|---|---|
| X2B1 | 2A2 | |
| T00 | 0 | 19327.7(3)c |
| A | 0.1893 | |
| ΔAd | 0.0073(5)c | |
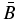 e e |
0.04848 | |
Δ e,d e,d |
−0.0017(1)c | |
| B – C | 0.01222 |
Notes.
aThe ground-state rotational constants were obtained by theoretical calculation of B3LYP/cc-pVTZ.
bThe excited-state constants were determined by rotational profile simulation as shown in Figure 2.
cValues in parentheses denote the uncertainties and apply to the last digits of the constants.
dΔA = A' − A, and  is also similar.
e
is also similar.
e = (B + C)/2.
= (B + C)/2.
Download table as: ASCIITypeset image
3.2. Simulation of Rotational Profiles with Rotational Distribution by Radiation and Collisions
A simulation of a rotational profile, which depends on the rotational distribution of molecules, is essential to compare a laboratory band with DIBs. In a diffuse cloud, where the number density is low, spontaneous emission between J and J − 1 is not negligible. The rotational distribution is determined based on their interaction with the environment through radiation and collisions. They are influenced by the radiative temperature Tr and the kinetic temperature Tk. When radiation is included along with collisions, the detailed balancing between J and J − 1 levels takes the form
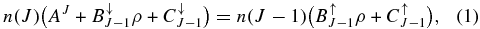
where n(J) is the number density of molecules with the rotational quantum number J, and Einstein A and B coefficients express spontaneous emission and induced radiative effect, respectively (Oka et al. 2013). The coefficients are expressed by
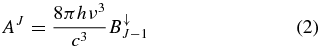
and
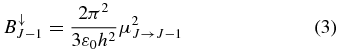
in the MKSA system, where ν is the transition frequency. The  and
and  constants in Equation (1) are the rates for collision-induced rotational transitions (Oka 1974) and can be approximated using Equation (7) in the report by Oka et al. (2013). Using Planck's formula
constants in Equation (1) are the rates for collision-induced rotational transitions (Oka 1974) and can be approximated using Equation (7) in the report by Oka et al. (2013). Using Planck's formula  and
and  , the relation between n(J) and n(J − 1) is derived as follows:
, the relation between n(J) and n(J − 1) is derived as follows:

(Oka et al. 2013).
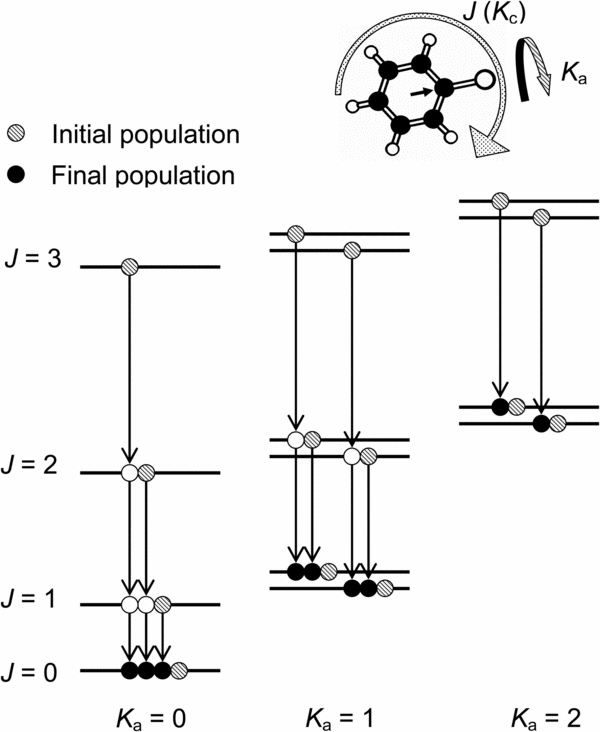
Consider the case of a near prolate C2v molecule. As the permanent dipole moment is along the a-axis, emission and absorption for rotational transitions are limited to the a-type transition, i.e., ΔKa is even and ΔKc is odd. As an assumption, we limited the transition to ΔKa = 0 and ΔKc = ±1 in the present analysis because the transitions of |ΔKa| ⩾ 2 are weak. Thus, transitions in each Ka level are similar to the case of a linear molecule, although J < Ka levels are missing, as shown in Figure 3. In this assumption, rotation along the a-axis cannot be cooled by radiation, and the a-axis can be regarded as a "hot axis." Rotation along the hot axis can result in a wide electronic transition profile due to the population of high Ka levels.
Figure 3. Schematic of the rotational energy levels and the presumed radiative population transfer of ΔKa = 0. The arrow in the ring of the thiophenoxy radical indicates the permanent dipole moment.
Download figure:
Standard image High-resolution imageIn the case of the J → J − 1 transition in each Ka series, the transition dipole is given as
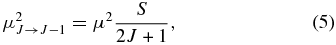
where μ is the permanent dipole moment and S is the transition strength. Using Equation (2) together with Equations (3), (5), and  in the ΔKa = 0 transition except for the Q-branch, we have
in the ΔKa = 0 transition except for the Q-branch, we have
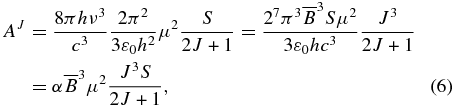
where α = 27π3/3ε0hc3. Thus, from Equations (4) and (6) we obtain
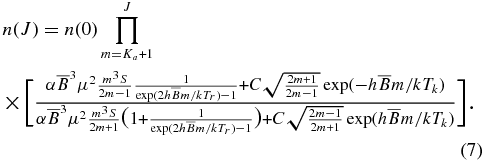
Since the number densities n(J) in our Equation (7) cannot be expressed analytically, we calculate them numerically. They are calculated as a function of the radiative temperature Tr (= 2.73 K) in a diffuse cloud due to the cosmic blackbody radiation, the kinetic temperature Tk, the rotational constant  , the permanent dipole moment μ, and the collision rate C.
, the permanent dipole moment μ, and the collision rate C.
At first, the population is distributed to each Ka series based on a simple Boltzmann population at the kinetic temperature Tk. Then, in each Ka series, relative populations for each J level are redistributed using our Equation (7). The calculated new distribution is input into PGOPHER, which can individually import the numerical population for each rotational level. The rotational profile is simulated using PGOPHER.
3.3. Simulation of Rotational Profiles of the Thiophenoxy Radical
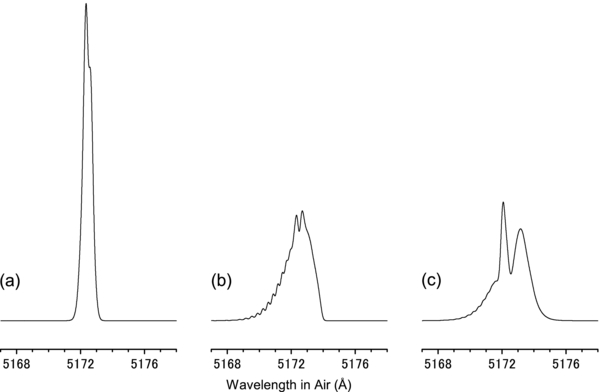
For simplicity, we employed a singlet approximation for the rotational structure, i.e., no spin splitting, to calculate the number densities n(J) for the thiophenoxy radical. The rotational constants and the permanent dipole moment obtained were  = 1453.5 MHz and μ = 3.1 D by the theoretical calculation of B3LYP/cc-pVTZ. The S values were derived from the rotational constant A, B, and C obtained from the calculation. In the diffuse clouds toward HD 204827, the excitation temperature of ∼40 K is presumed from the rotational structure of the C2 spectrum (Oka et al. 2003). As the excitation temperature of a molecule with no permanent dipole moment can correspond to the kinetic temperature, we used Tk = 40 K toward HD 204827. The collision rate was assumed to be C = 10−7 s−1 as a typical value of diffuse clouds (Oka & Epp 2004; Oka et al. 2013). Toward HD 204827, the K I line is resolved into at least four components (Pan et al. 2004), and the two strong components are separated by approximately 5 km s−1, i.e., 0.09 Å at the 7172 Å region. The reported DIB spectrum (Hobbs et al. 2008) was obtained by the resolving power R = 38,000, which gave a resolution of 0.14 Å at 7172 Å. To estimate the rotational profile as a DIB toward HD 204827, we used a resolution of 0.22 Å as a summation of the two components' separation and the resolution. The thiophenoxy radical was simulated for Tk = 40 K and Tr = 2.73 K, hereafter case (b), as shown in Figure 4(b). For comparison, the profiles with the extreme cases, Tk = Tr = 2.73 K (case (a)) and the simple Boltzmann population at 40 K (case (c)), were also simulated, as shown in Figures 4(a) and (c), respectively. Not only case (c) but also case (b) shows a wide width for the profile, although the structures are not similar.
= 1453.5 MHz and μ = 3.1 D by the theoretical calculation of B3LYP/cc-pVTZ. The S values were derived from the rotational constant A, B, and C obtained from the calculation. In the diffuse clouds toward HD 204827, the excitation temperature of ∼40 K is presumed from the rotational structure of the C2 spectrum (Oka et al. 2003). As the excitation temperature of a molecule with no permanent dipole moment can correspond to the kinetic temperature, we used Tk = 40 K toward HD 204827. The collision rate was assumed to be C = 10−7 s−1 as a typical value of diffuse clouds (Oka & Epp 2004; Oka et al. 2013). Toward HD 204827, the K I line is resolved into at least four components (Pan et al. 2004), and the two strong components are separated by approximately 5 km s−1, i.e., 0.09 Å at the 7172 Å region. The reported DIB spectrum (Hobbs et al. 2008) was obtained by the resolving power R = 38,000, which gave a resolution of 0.14 Å at 7172 Å. To estimate the rotational profile as a DIB toward HD 204827, we used a resolution of 0.22 Å as a summation of the two components' separation and the resolution. The thiophenoxy radical was simulated for Tk = 40 K and Tr = 2.73 K, hereafter case (b), as shown in Figure 4(b). For comparison, the profiles with the extreme cases, Tk = Tr = 2.73 K (case (a)) and the simple Boltzmann population at 40 K (case (c)), were also simulated, as shown in Figures 4(a) and (c), respectively. Not only case (c) but also case (b) shows a wide width for the profile, although the structures are not similar.
Figure 4. Simulated rotational profiles of thiophenoxy radical for case (a) Tk = Tr = 2.73 K, (b) Tk = 40 K and Tr = 2.73 K, and (c) the simple Boltzmann population at 40 K.
Download figure:
Standard image High-resolution imageNext, we considered the contribution of the ΔKa = ±2 (ΔKc = ±1) transitions, which can transfer population from high Ka to low Ka by radiative cooling in Tr < Tk. The ν3S values of the ΔKa = ±2 transitions in this molecule are smaller by one order than those of the ΔKa = 0 transitions; thus, the values of AJ(ΔKa = ±2) are not so far from that of the collision rate C. This radiative cooling by the ΔKa = ±2 transitions may compete with collisional heating. However, a simulation including the cooling and the heating for ΔKa = ±2 is difficult because a proper value of C is difficult to estimate. The case (b) simulation can be suitable if the relation C ≫ AJ(ΔKa = ±2) holds. The actual rotational profile of the DIB may be between case (a) and case (b) in Figure 4.
The observed electronic transition of the thiophenoxy radical (5173 Å in air) lies near two reported DIBs at 5170 and 5176 Å (Hobbs et al. 2008). To check whether the small wavelength differences are an effect of rotational structure, a comparison was made between a DIB spectrum in the 5150–5192 Å range and the simulated spectrum of the thiophenoxy radical in case (b). The results are shown in Figure 5, and it is clear that the simulated spectrum does not fit the DIB absorptions for both width and wavelength.
Figure 5. Comparison of the simulated rotational profile of the thiophenoxy radical in case (b) with DIB spectrum toward HD 204827 (Hobbs et al. 2008). In the upper trace, three bands marked ○ are DIBs and the other lines are stellar.
Download figure:
Standard image High-resolution imageAn upper limit of the column density for the thiophenoxy radical toward HD 204827 can be estimated using the procedure used by Motylewski et al. (2000). It was assumed that a signal-to-noise ratio of 5 is required to detect DIB absorption. The FWHM of the band is approximately 2 Å, as shown in Figure 4(b), and the detection limit of the equivalent width in the data presented by Hobbs et al. (2008) is 0.015 Å. The oscillator strength of the 2A2 – X2B1 transition for the thiophenoxy radical that is theoretically obtained by TD-B3LYP/cc-pVTZ is 0.003. These values then lead to 2 × 1013 cm−2 as the upper limit of the column density for the thiophenoxy radical toward HD 204827. This upper limit is relatively larger than that of C6H ((1.09–1.43) × 1012 cm−2) reported by Motylewski et al. (2000) because of the small oscillator strength of the thiophenoxy radical. The two transitions from the ground state to the third (2B1) and fifth (2B1) excited states have strong oscillator strengths of 0.057 and 0.054, respectively, obtained theoretically. The two transitions may correspond to the broad emission band at the 4000–5000 Å region in the solution reported by Russell (1975) and the absorption band at ∼3100 Å in the vapor phase reported by Porter & Wright (1955). However, these reported bands are not high resolution. Since the line width of a typical DIB is ∼1 Å, the transition to the long-lifetime excited state can be a candidate for an origin of the typical DIB. To have a lifetime broadening of <1 Å at 4000 Å, the excited-state lifetime of >1 ps is necessary. The lifetime broadening of the observed band in this work as shown in Figure 2 is less than ∼0.1 cm−1, which corresponds to the lifetime of >50 ps. To compare the two transitions with DIBs and to determine the upper limits of the column densities of the two transitions, high-resolution observations of laboratory spectra that can show magnitudes of lifetime broadening are necessary.
The rotational profile of a linear molecule is correlated with the radiative temperature Tr in a diffuse cloud as reported by Oka et al. (2013). In this study, it is suggested that the rotational profile of the near prolate C2v molecule can depend not only on the radiative temperature but also on the collision rate due to the non-negligible ΔKa = ±2 transitions. Band widths of some DIBs depend on the line of sight (e.g., Oka et al. 2013). It is possible that a near prolate C2v molecule with a hot axis is a potential molecular origin of a DIB with such line-of-sight dependence, because the collision rate varies depending on a cloud.
3.4. Summary
The thiophenoxy radical was produced in the hollow cathode glow discharge of thiophenol. The 2A2 – X2B1 electronic transition of the thiophenoxy radical was observed using a newly constructed cavity ringdown spectrometer. To compare the observed laboratory data with the reported DIB toward HD 204827 (Hobbs et al. 2008), we derived the model of rotational distribution by radiation and collisions for the near prolate C2v molecule in a diffuse cloud. The rotational profile of the thiophenoxy radical as a DIB was simulated by the model. Although the comparison with the reported DIB suggests no agreement, the upper limit of the column density was estimated to be 2 × 1013 cm−2. The reported profile simulation raises the possibility that DIB with a variable line width dependent on the line of sight originates from a near prolate C2v molecule.
This study was funded by the Tokyo Ohka Foundation for the Promotion of Science and Technology, the Research Foundation for Opto-Science and Technology, the Sumitomo Foundation, and Grant-in-Aid for Scientific Research on Innovative Areas (grant No. 25108002).