Abstract
The impacts of the progress in the art and science of crystal growth on human life are reviewed. Even before the invention of the transistor, quartz and corundum crystals were used as crystal oscillators and jewel bearings, respectively. However, a major impact of crystal growth on society was experienced with the invention of the transistor, which required high-purity and perfect germanium crystals. Once the importance of crystal growth was clearly recognized, the science of crystal growth also extensively developed. The growth of single crystalline silicon allows us to produce integrated circuits, which are used in all the electronic devices in everyday use. The technological developments in the growth of compound semiconductors have also had a large impact on society through the inventions of the laser diode for optical communication and the p–n junction nitride light-emitting diode toward the realization of a less energy-intensive society. The latter invention was awarded the 2014 Nobel Prize in Physics. Finally, future aspects of crystal growth are discussed.
Export citation and abstract BibTeX RIS
1. Introduction
Modern society might not exist without the progress in the art and science of crystal growth, especially that of semiconductors. Large-scale silicon integrated circuits, which are made of silicon single crystals, appear in all kinds of computers from laptops to supercomputers. Information and telecommunication systems employ semiconductor lasers and high-speed compound semiconductor transistors as well as silicon integrated circuits. Silicon integrated circuits and semiconductor light-emitting devices are also found in mobile telephones, televisions, audio equipment, refrigerators, washing machines, and other electronic appliances used in daily life. Furthermore, silicon integrated circuits, semiconductor power elements, and semiconductor optical devices are now used as the key parts of automobiles, which have typically been considered as mechanical products. The same trend has been occurring in aircrafts, ships and other vehicles. Traffic lights, room illumination, and advertising display panels are now employing semiconductor light-emitting diodes (LEDs). Semiconductor solar cells are now one of the key components expected to lead to a future low-energy society.
Most of these semiconductor devices are made of single crystals. Looking back at the history of new semiconductor devices, they are always preceded by the invention of new crystal growth technology. As we will see in the following sections, the germanium transistor would not have been invented without the growth of high-purity germanium crystals. Behind the great advances in silicon integrated circuits was the development of new technology enabling the growth of large-scale dislocation-free silicon single crystal. The invention of liquid-phase epitaxy made it possible to realize semiconductor laser diodes (LD) and commercial LEDs. Drastic technological advances in heteroepitaxy with a large lattice mismatch led to the realization of p–n junction nitride LEDs and LDs. The impacts of crystal growth on the invention of new devices are summarized in Fig. 1. A detailed explanation of the contents of the table will be given in the following sections.
Fig. 1. Historical events in crystal growth and their impacts on the invention of new devices.
Download figure:
Standard image High-resolution imageFigure 1 illustrates only some examples showing how technological developments in crystal growth have made it possible to create new devices that have changed society. In contrast to the old art of crystal growth, modern crystal growth technology is based on the advanced science of crystal growth. Crystallography is one of the fields closest to crystal growth. Scientists in crystallography have made great effort to clarify the static characteristics of crystal surfaces and bulks using various sophisticated tools such as X-ray diffraction, electron microscopy, scanning tunneling microscopy, and so forth. On the other hand, researchers of crystal growth study the dynamical behavior of atoms and molecules on the surface of a growing crystal. Therefore, to understand crystal growth, one should employ tools such as thermodynamics, statistical physics, and quantum mechanics together with up-to-date knowledge of crystallography.
2. Brief historical view of impacts of crystal growth on society
2.1. Quartz
Quartz crystals have a long history of being used as crystal oscillators, which give an accurate oscillation frequency that can be used for telecommunication systems. Since the invention of the transistor, crystal oscillators have been used in watches, clocks, computers, mobile phones, and so forth. Up to the end of World War II, naturally grown quartz crystals were used. However, to meet the demand for them, the hydrothermal synthesis of quartz crystals was developed for their large-scale production, and all crystals are now grown artificially.1–4)
The method used for the hydrothermal growth of quartz is schematically illustrated in Fig. 2. Crystal growth is conducted in an aqueous solution of NaOH or Na2CO3 under high pressure and high temperature. In the upper zone, the temperature is kept slightly lower than that in the lower zone. The source quartz crystals are dissolved in the solution and transported to the upper zone, where growth occurs on seed crystals. By increasing the number of seed crystals, one can grow a large number of high-quality quartz crystals. An example of an as-grown quartz crystal is shown in Fig. 3.
Fig. 2. Schematic illustration of the high-pressure furnace for the hydrothermal growth of quartz. The temperatures of the seed and source zones are kept respectively at approximately 350 and 400 °C. The baffle shown in the figure is used to control the flow in the solution.
Download figure:
Standard image High-resolution imageFig. 3. As-grown quartz crystal obtained by hydrothermal method. In mass production, such crystals are grown simultaneously and the total weight of grown crystals is sometimes more than 1 ton per run.
Download figure:
Standard image High-resolution image2.2. Ruby and sapphire
Ruby and sapphire, which belong to a group of corundum crystals, also have a long history of being used as jewel bearings. They are used in mechanical watches, and high-accuracy measurement instruments such as galvanometers and gyroscopes. The Verneuil process developed in 1902 has been employed to grow these crystals, and a modified process for mass production is still used at present.5)
A schematic illustration of the Verneuil technique is given in Fig. 4. The source material is Al2O3 powder, which is placed in the upper container. The powder is sent to the seed crystal in the growth chamber through a tube with oxygen gas. As shown in the figure, hydrogen gas is supplied through the outer tube to the growth chamber, where both gases react to give a high-temperature flame. The flame heats both the powder and the surface of the seed crystal to form a melt. By pulling down the seed crystal, continuous growth can be achieved. Corundum crystals are also grown by the Czochralski method.6)
Fig. 4. Schematic illustration of Verneuil furnace. Al2O3 powder is supplied to the growth chamber with oxygen gas. Hydrogen gas, which is introduced through the outer tube, reacts with the oxygen to give a high-temperature flame, which heats both the Al2O3 power and the surface of the seed to form a melt, and the growth is conducted by pulling down the grown crystal. By adding chromium oxide to the Al2O3 power, ruby is obtained. The corundum crystal other than ruby is called sapphire.
Download figure:
Standard image High-resolution image2.3. Germanium
In human history, the greatest impact of crystal growth on industry was the invention of the point contact transistor in 1947 and the junction transistor in 1948. The action of a transistor would never have been observed without the realization of a single crystal growth of germanium with high purity. Bell Telephone Laboratories had a long history of growing single crystals of halides7) and metals. The art and science of these growth technologies were applied to grow single crystals of germanium. After the invention of point contact and junction transistors, the systematic development of growth and purification technologies was greatly required, and the Bridgman and Czochralski techniques were applied to grow single crystals of germanium. A schematic illustration of the Czochralski method is given in Fig. 5. After melting the material, a thin seed is brought in contact with the surface of the melt, and then the crystal is pulled upward to start the growth.
Fig. 5. Schematic illustration of Czochralski method.
Download figure:
Standard image High-resolution imageThe purification of germanium was another key technology required to produce transistors. Pfann developed the zone-melting technique, which enabled the purification of crystals and also the doping of impurities to a predetermined level.8,9) The invention of the transistor was the first epoch-making event that clearly showed how crystal growth is important for industry and society. After the success of germanium point contact and junction transistors, germanium took the position of the dominant material in solid-state electronics. However, silicon soon came to take this position. Since silicon cannot be grown by the Bridgman technique owing to its reaction with the crucible, the Czochralski technique was employed.
2.4. Silicon
The first successful growth of a silicon single crystal by the Czochralski method was accomplished by Teal and Buehler in 1949 at Bell Telephone Laboratories.10,11) At that time, the size of grown silicon crystals was 1 in. in diameter and 5 in. in length. The development of the Czochralski technique allowed the growth of dislocation-free silicon crystals with the diameter of 16 in. From such large-scale silicon wafers, silicon integrated circuits are produced and used in computers, cell phones, cars, and many other products.
The next epoch-making event in the crystal growth of semiconductors was the introduction of the use of epitaxial growth for devices. Theuerer of Bell Telephone Laboratories succeeded in growing a thin single-crystal film of silicon epitaxially on a silicon substrate,12) while his group was trying to grow a high-resistivity film on a low-resistivity substrate for mesa transistors and other devices. Figure 6 schematically illustrates typical apparatus used for silicon vapor-phase growth. A mixture of hydrogen and SiCl4 is sent to a silicon substrate heated by a radio-frequency (RF) electromagnetic wave. At a high temperature, SiCl4 is reduced by hydrogen resulting in the growth of silicon atoms on the substrate. The growth temperature is between 1000 and 1300 °C. By epitaxial growth, one can reduce the collector resistivity of n–p–n planar transistors as shown in Fig. 7. The epitaxial growth of silicon has provided the freedom to design various types of silicon devices including integrated circuits.
Fig. 6. Vapor-phase epitaxial growth by hydrogen reduction of SiCl4. Prior to the growth, the Si substrate is etched by HCl at an elevated temperature to remove a natural oxide layer.
Download figure:
Standard image High-resolution imageFig. 7. Structure of a planar transistor. To produce a n–p–n structure by impurity diffusion, the collector layer should have high resistivity. By employing epitaxy, one can decrease the thickness of the collector region, as shown in the figure, and decrease the collector resistivity.
Download figure:
Standard image High-resolution image2.5. Compound semiconductors
2.5.1. Liquid-phase epitaxy
The invention of liquid-phase epitaxy (LPE) by Nelson at RCA Laboratories led to a breakthrough in the crystal growth of compound semiconductors.13) The principle of GaAs LPE is shown schematically in Fig. 8. LPE is conducted in a graphite boat of high purity, which is surrounded by high-purity hydrogen gas. Before the growth, the sliding part of the boat (slider) is at position (a) and GaAs is saturated with high-purity gallium metal contained in the well of the slider at an elevated temperature. After the saturation is completed, the slider is moved on the substrate to take position (b). Then, the temperature is decreased to start the growth. The temperature is continuously decreased to maintain growth at a certain rate. After the required thickness of GaAs is attained, the slider is moved back to position (a) so that the growth is terminated. For the growth of many layers with different types of conductivity and alloy compositions, a slider with many wells is used.
Fig. 8. Schematic illustration of slide boat system used for LPE. A slider is placed at position (a) to ensure the saturation of GaAs in Ga metal at high temperatures, and then it is moved to the position (b) to induce growth upon decreasing the temperature.
Download figure:
Standard image High-resolution imageIn LPE, the growth is usually carried out under a condition quite close to equilibrium, which is extremely important for obtaining high-quality layers with a low concentration of point defects and a stoichiometric composition. The invention of LPE was a key factor leading to the successful fabrication of LDs and commercial optoelectronic devices such as LEDs. LDs operating at room temperature and in continuous-wave mode were made possible by this invention. Alferov succeeded in growing an LD with a GaAs–GaAlAs double heterostructure by LPE, for which he was awarded the Novel Prize in Physics in 2000.
The successful use of LPE in industry has promoted advances in the science of epitaxial growth and greater understanding of the growth mechanism of LPE. Figure 9 shows the growth spiral found on an as-grown surface of InP after LPE by atomic force microscopy (AFM).14) It has been found that the interstep distance of a growth spiral is given by15)

where γ', νs, m, and Δμ are the free energy of the side surface of the spiral step, the molar volume of the growing species, the number of spirals at the center, and the difference in the chemical potential between the growth atmosphere and the crystal, respectively. Δμ is given by
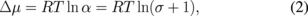
where α is the supersaturation ratio and σ is the supersaturation. Hence, once the interstep distance of the growth spiral is measured by AFM or differential interference contrast microscopy, one can obtain the value of the supersaturation in the vicinity of the growing surface. The interstep distance near the spiral center in Fig. 9 was measured to be 0.93 µm and the supersaturation was calculated to be 0.05 with a tentative value of γ' = 0.12 J/m2.14) The absolute value of the supersaturation is not very reliable because the value of γ' is calculated using the broken bond model and the latent heat of InP. However, the change in the supersaturation upon changing the growth parameters can be reliably predicted, and this gives important information to clarifying the growth mechanism.
Fig. 9. AFM image of spiral step observed on the surface of InP grown by LPE. From the step distance near the center, one can calculate the supersaturation, which in this case is 0.05 (see text).14)
Download figure:
Standard image High-resolution imageIn general, it is very difficult to measure the supersaturation on a surface during growth. However, by this method, one can find the supersaturation as a function of growth temperature and other growth parameters. The successful measurement of the supersaturation on a growing surface was a major achievement that had not previously been obtained in semiconductor epitaxy, and this method has been applied to find the supersaturation in GaN epitaxy, which will be discussed later.
Another important achievement in semiconductor growth science was discovering the formation mechanism of macrosteps. It is well-known that macrosteps appear on a surface after vapor growth, melt growth, and solution growth. They are generated on the growing surface due to step bunching. In solution growth such as LPE, it was found that the bunching occurs as a result of a morphological instability.16,17) This has been confirmed by micro-gravity experiments conducted in a space shuttle.18–20)
It was also found that the impurity nonuniformity is induced in association with macrostep risers.21) Figure 10 shows a surface micrograph of LPE-grown GaP (a) and a photoluminescence image of the cleaved cross section (b). One clearly sees that the dark lines are formed under macrostep risers.20) It was found that the dark lines appear because the nitrogen concentration is low in the region where the luminescence is weak.22) Nitrogen takes a substitutional site in GaP and acts as a luminescence center.
Fig. 10. Optical micrograph of GaP(111) surface doped with nitrogen grown by LPE (a). Photoluminescence image of cleaved cross section of the crystal (b). The dark areas, in which the nitrogen concentration is low, are formed under macrostep risers.20)
Download figure:
Standard image High-resolution imageFigure 11(a) shows a surface micrograph of GaP grown by LPE taken by a differential interference contrast microscope.23) In the figure, macrosteps and atomistic steps can be seen, the latter of which are generated by screw dislocations. An overall picture of the growth is given in Fig. 11(b). The macrosteps move slowly during the growth and atomistic steps run along the terraces of the macrosteps. The vertical growth is carried out by the propagation of the atomistic steps from the screw dislocations.
Fig. 11. Surface micrograph of a GaP LPE layer taken by differential interference contrast microscope (a). The bold black arrow shows the moving direction of macrosteps and the white arrow shows the direction of atomistic steps propagating on the growth surface.23) (b) Schematic illustration of the overall picture of the growth. After the formation of macrosteps by step bunching, the growth steps are supplied from screw dislocations.
Download figure:
Standard image High-resolution image2.5.2. Molecular beam epitaxy and metalorganic chemical vapor deposition
The next major breakthrough made in epitaxial technology was the birth and development of molecular beam epitaxy (MBE) and metalorganic chemical vapor deposition (MOCVD). Cho (Bell Telephone Laboratories), and Manasevit and Simpson (North American Rockwell) respectively reported the growth of GaAs by MBE24) in 1970 and of GaAs, GaP, and their alloys by MOCVD25) in 1969. The MBE chamber designed by Cho in 1970 is illustrated in Fig. 12. Since the beginning of MBE, all the elements equipped in modern MBE systems, such as a liquid nitrogen shroud, a Knudsen cell, and a reflection high-energy electron diffraction (RHEED) system, were installed. The great advantage of MBE is the possibility of real-time monitoring of the growth process. Among the many in situ monitoring systems, the RHEED system is most powerful and has become an indispensable tool. In 1981, Harris et al. found that during the growth of GaAs by MBE, the intensity of the RHEED image oscillates with an oscillation period identical to the time for GaAs monolayer growth.26)
Fig. 12. Schematic picture of MBE system employed by Cho in 1970.24)
Download figure:
Standard image High-resolution imageFigure 13 shows the RHEED intensity oscillation during the growth of GaAs by MBE observed by Neave et al.27) The intensity oscillation is explained as follows. After keeping a GaAs substrate at a high temperature under an arsenic flux, the surface becomes very flat and the RHEED intensity takes a maximum value. However, when the growth starts, the intensity decreases because the surface starts being covered by two-dimensional nuclei and the electrons are scattered in random directions.
Fig. 13. RHEED intensity oscillation observed by Neave et al.27)
Download figure:
Standard image High-resolution imageThe RHEED oscillation is extremely useful. First, it is used to monitor the thickness of the growth layer. Control of the thickness with monolayer accuracy is often required in the fabrication of devices such as LDs, quantum well devices, and high-electron mobility transistors (HEMTs). Secondly, it can be used to detect the birth and spread of two-dimensional nuclei on the growing surface. By utilizing this principle, it was found that there are two modes of growth in MBE.28,29) One is the step-flow mode, in which the growth is conducted by the advance of the step train in one direction without nucleation. The other is the two-dimensional nucleation mode, in which the growth is carried out by the steps generated by nuclei. Thus, RHEED oscillation is an indispensable tool for understanding the growth mechanism. This is another example of how the technological development of crystal growth has contributed to the advance of crystal growth science.
MOCVD is another important tool for epitaxy. In contrast to LPE and halogen vapor-phase epitaxy, in both MBE and MOCVD there is almost no chemical reaction by which the substrate is dissolved during the growth. This means that one can achieve growth with a very sharp interface. In the early development of MOCVD by Manasevit and his group, there was a purity problem with the metalorganic sources. However, the purity was improved drastically in the 1970's, enabling high-purity and high-quality films to be used for all devices. MOCVD is now the most important method in industry for growing nitride semiconductors.
Figure 14 shows a schematic diagram of MOCVD apparatus for the growth of GaAs. The metalorganic source can be triethylgallium instead of trimethylgallium. If one wishes to grow AlAs or GaAlAs, one can employ a trimethyl- or triethylaluminum source in addition. When the doping of an impurity is necessary, one adds supply lines to provide metalorganic zinc and hydrides of sulfur and selenium, for instance. By controlling the flow rate very accurately, the growth rate can be kept very low similarly to in MBE. Hence, MOCVD can be used for growing quantum well lasers, HEMTs, and other sophisticated devices.
Fig. 14. Schematic illustration of MOCVD system.
Download figure:
Standard image High-resolution imageOne of the largest contributions of MOCVD to growth science has been to establish the relationship between the growth rate and surface supersaturation. Akasaka et al. employed the selective-area epitaxy (SAE) of GaN to separate areas with a screw dislocation from those without.30) Then, they measured surface supersaturation from the interstep distance of spiral steps in the area with a screw dislocation using Eq. (1). The growth rate was obtained by measuring the height of the area after the growth.31)
Figure 15 shows a growth spiral observed by Akasaka et al. by AFM.32) It is seen in the figure that two spiral steps are generated at the center and hence m in Eq. (1) is 2. Figure 16 gives the growth rate as a function of supersaturation calculated by Eq. (1).32) In the figure, the curved line gives the theoretical growth rate calculated by Akasaka et al. using Burton–Cabrera–Frank (BCF) theory and closed diamonds show the experimental values. According to BCF theory, the growth rate is proportional to σ2 in the low-σ region and proportional to σ in the high-σ region. The boundary between the two regions is calculated as σ1 = 0.5 in this case.32) As seen in the figure, all experiments were conducted in the low-σ region and the growth rate follows a quadratic function of σ, in good agreement with the theory. On the other hand, the growth rate in the area with no screw dislocations is very low in this range of supersaturation and increases marginally at the highest supersaturation in the experiment.32)
Fig. 15. AFM image of spiral steps observed on MOCVD-grown GaN surface by Akasaka et al.32)
Download figure:
Standard image High-resolution imageFig. 16. Experimental growth rate vs supersaturation obtained by Akasaka et al.32) In this range of supersaturation, growth by two-dimensional nucleation (closed circles) is much slower than that by spiral steps (closed diamonds).
Download figure:
Standard image High-resolution imageBCF theory assumes that the growth is conducted by steps from screw dislocations and that the surface diffusion is a rate-limiting process. MOCVD is one of the best methods for the application of BCF theory. In the history of crystal growth theory, BCF theory has been applied to solution growth, but in solution growth it is not clear whether surface diffusion plays an important role. In this respect, the MOCVD conducted by Akasaka et al. is one of the best examples to which BCF theory can be applied.
3. Highly lattice mismatched heteroepitaxy
After the success of the LD with a double heterostructure grown by LPE, the guiding principle for obtaining a high-quality layer of heteroepitaxy was to find a heteroepitaxial combination with as small lattice mismatch as possible. GaAs–GaAlAs is ideal because the lattice mismatch is almost zero. However, there are many heteroepitaxial combinations that are strongly required to use even though the lattice mismatch is large. The heteroepitaxy of GaAs on silicon is one such example. Silicon is the most suitable material for fabricating integrated circuits while GaAs is an excellent material for optical devices. Hence, if one can grow high-quality GaAs on a silicon substrate, one can fabricate optoelectronic integrated circuits (OEICs).
Another example of heteroepitaxy on a large mismatched substrate is the growth of GaN on a foreign substrate. Since the bulk crystal growth of GaN is very difficult, large GaN wafers are not available at present. Hence, a single-crystal substrate of another material such as sapphire or SiC is usually used.
However, in such heteroepitaxy there is a large lattice mismatch at the heterointerface, which results in the generation of a large number of defects. To overcome this problem, a technology was strongly required to grow a high-quality epitaxial layer on a substrate with a large lattice mismatch. This technology was called HM2 (highly lattice mismached heteroepitaxy).33)
When HM2 is conducted, sometimes the heteroepitaxial layer rotates crystallographically to decrease the interfacial energy and takes the position of a coincidence site lattice (CSL).34,35) For instance, it is well known that GaN grown on (0001) sapphire takes the crystallographic relations (0001)GaN ∥ (0001)sapphire and ![$[1\bar{1}00]_{\text{GaN}}\parallel [11\bar{2}0]_{\text{sapphire}}$](https://content.cld.iop.org/journals/1347-4065/54/5/050101/revision1/IR140003if001.gif) . The CSL arrangement reduces the lattice mismatch to 15% from 32%, which is the mismatch when both a-axes align in the same direction. It has also been demonstrated that AlN grown on silicon takes the CSL arrangement of (0001)AlN ∥ (111)Si and
. The CSL arrangement reduces the lattice mismatch to 15% from 32%, which is the mismatch when both a-axes align in the same direction. It has also been demonstrated that AlN grown on silicon takes the CSL arrangement of (0001)AlN ∥ (111)Si and ![$[\bar{2}110]_{\text{AIN}}\parallel [\bar{1}10]_{\text{Si}}$](https://content.cld.iop.org/journals/1347-4065/54/5/050101/revision1/IR140003if002.gif) .36)
.36)
To artificially reduce the density of defects generated at the heterointerface, there are three HM2 technologies: (1) two-step growth, (2) the utilization of a superlattice, and (3) microchannel epitaxy.
3.1. Two-step growth
Two-step growth consists of initial growth at a low temperature and successive growth at a higher temperature. At the low temperature, a thin buffer layer of poor crystal quality is grown on the substrate, and then the growth is carried out at the normal higher growth temperature. The idea is to confine a large number of misfit dislocations generated at the interface within the buffer layer.
Looking back at the history of two-step growth, in 1978, Ohnishi et al. reported deposition of a ZnO buffer layer of 13 nm thickness at 100–160 °C on a sapphire substrate by sputtering followed by the growth of ZnO at 900–1000 °C by CVD. They obtained a ZnO epitaxial layer of high quality.37) Then, in 1980, Nishino et al. used two-step growth to grow SiC on a silicon substrate.38) They deposited a SiC buffer layer on a silicon substrate at 800 °C by RF sputtering and grew SiC of good quality at 1360 °C by CVD.
Remarkable success was attained in growing a high quality silicon epitaxial layer on a sapphire substrate by Ishida et al. in 1981.39) They deposited an amorphous silicon film on a sapphire substrate by sputtering at room temperature. The thickness of the buffer layer is important in determining the quality of the successively grown layer and they reported that it should be 2–4 nm. The growth of silicon in the second step was conducted at 1000 °C. The quality of the film was excellent and the MOSFET fabricated with this film showed very good properties.40)
Two-step growth was also applied to the heteroepitaxy of GaAs on silicon by Akiyama et al. in 1984.41) To avoid the formation of an antiphase domain, a vicinal (001) substrate was employed. They grew GaAs of good quality by both MOCVD and MBE and reported an etch pit density on the order of 103/cm2. However, in their case, it turned out that the etch pits corresponded to bunched dislocations and the density of individual dislocations was on the order of 106/cm2, which is excellent even for HM2.
The most successful application of two-step growth was attained in the growth of nitride semiconductors. Akasaki's group succeeded in growing high-quality GaN on sapphire using an AlN buffer layer in 1986.42) The grown crystal was optically flat without cracks, which had never been achieved by other methods. Figure 17 shows the effect of a low-temperature buffer layer on the crystal quality of GaN.43,44) Figures 17(a) and 17(b) present surface micrographs of GaN grown on sapphire without a low-temperature buffer layer, taken using transmission and reflection light, respectively. Figure 17(c) gives an illustration of the cross section of GaN layer directly grown on sapphire, where pits, cracks and highly conductive regions are schematically shown. Figure 17(d) presents GaN crystal grown at a high temperature on the AlN buffer layer grown at a low temperature on a sapphire substrate. The film is transparent and shows a very sharp X-ray diffraction peak and strong photoluminescence band edge emission.
Fig. 17. Marked improvement of GaN quality after using low-temperature buffer layer. (a) and (b) Surface micrographs of GaN grown on sapphire without a low-temperature buffer layer taken using transmission and reflection light, respectively. (c) Schematic illustration of the cross section. (d) GaN crystal grown on a low-temperature buffer layer.43,44)
Download figure:
Standard image High-resolution imageHigh-quality AlGaN was also grown by this method.45,46) Then in 1989, the same group succeeded in obtaining p-type GaN by doping Mg and activating it by electron beam irradiation, then they fabricated a p–n junction for the first time in the history of nitride semiconductors.47,48) The success of the p–n junction made it possible to produce efficient blue to ultraviolet LEDs and LDs, and it can be said that their work gave blue light to the world. Akasaki and Amano were awarded the 2014 Nobel Prize in Physics for the invention of the p–n junction blue LED.
In 1992, Nakamura et al. found that p-type GaN could be obtained by the thermal activation of Mg-doped GaN, which was also grown with a low-temperature buffer layer.49) Thermal activation is ideally suited to the mass production of nitride devices and Nakamura was jointly awarded the 2014 Nobel Prize in Physics due to the invention for obtaining the p-type GaN by thermal activation. Blue and white LEDs are now ubiquitous in villages, towns, and cities worldwide. This is the best example of how a development in crystal growth technology, in this case the use of a low-temperature buffer layer, has changed society.
3.2. Utilization of a superlattice
Superlattices have been employed to reduce the density of misfit dislocations generated at a heteroepitaxy interface. Superlattices are known to block the propagation of threading dislocations as shown by Matthews et al. as early as in 1976.50) In 1984, Fischer et al. employed a GaAs/GaAlAs superlattice to reduce the dislocation density in GaAs heteroepitaxially grown on a silicon substrate.51)
Umeno's group grew GaAs of good quality on a silicon substrate with an AlP, AlGaP, GaP/GaAs0.5P0.5, superlattice and a GaAs0.5P0.5/GaAs superlattice.52) In their case, the superlattice consisted of layers with different lattice constants, which induced a strain in the layers. By employing a superlattice, one can decrease the dislocation density of GaAs on a silicon substrate to the order of 106/cm2, but it is not possible to eliminate dislocations completely. To fabricate OEICs, the dislocation density should be nearly zero so that no dislocations remain in the active layer of the LD. However, if the dislocation density is not a major constraint for devices, such as nitride-based devices on silicon, one can use a superlattice as a buffer layer to reduce the dislocation density.
3.3. Microchannel epitaxy
The idea of microchannel epitaxy (MCE) is shown in Fig. 18.53) As seen in Fig. 18(a), in ordinary epitaxy the grown layer becomes single crystalline by inheriting the lattice information of the substrate. However, if a dislocation penetrating the surface is present in the substrate, the dislocation will be inherited in the epitaxial layer.
Fig. 18. Conventional epitaxy (a) and MCE (b, c).53) In conventional epitaxy, both lattice and defect information is inherited to the epitaxial layer. However, in MCE the lattice information is transmitted through the microchannel but defect information is blocked by the amorphous film. The MCE in (b) and that in (c) have been named as HMCE and VMCE, respectively. For simplicity, HMCE, which is much more frequently employed, is referred to MCE.
Download figure:
Standard image High-resolution imageThere are two ideas for preventing the propagation of dislocations. The first one is shown in Fig. 18(b). An amorphous film such as SiO2 is deposited on the substrate and a narrow window called a microchannel is cut in the amorphous film. When the growth starts inside the microchannel, it then proceeds laterally over the amorphous film. Since the amorphous film is present between the substrate and the laterally grown layer, a dislocation in the substrate cannot propagate into the grown layer as shown in the figure. Hence, if no threading dislocations are present in the microchannel area, the grown layer becomes dislocation-free. The main idea is that although lattice information is inherited through the narrow microchannel to realize epitaxial growth, defect information is blocked by the amorphous film. This type of MCE has been named as horizontal MCE (HMCE).53)
The second idea of MCE is given in Fig. 18(c). In this case, the growth is conducted in the vertical direction through the microchannel. The grown layer can be a slab or a rod. This idea of MCE has been named as vertical MCE (VMCE).53) Depending on the crystallographic orientation of the substrate, a dislocation often takes an off-normal orientation relative to the substrate surface. Then, the dislocation exits the epitaxial layer from the side of the layer as shown in the figure. Since HMCE is much more frequently used than VMCE, hereafter, we refer to MCE instead of HMCE for simplicity.
Figure 19 schematically illustrates the MCE of GaAs on a silicon substrate. To realize large lateral growth, LPE is employed. LPE has the advantage of growth with very low supersaturation, which enables large growth anisotropy to be realized, namely, a large ratio of the horizontal width to the vertical thickness. First, a GaAs buffer layer is grown by MBE or MOCVD on a silicon substrate, then the LPE growth of GaAs is conducted through a microchannel opened in an amorphous film, in this case, a SiO2 film.54–57)
Fig. 19. Schematic cross section of MCE of GaAs on silicon substrate.54)
Download figure:
Standard image High-resolution imageAn optical micrograph of MCE GaAs grown on silicon is shown in Fig. 20. In the central region, an area of dislocations with a width of approximately 25 µm can be seen. A large number of dislocations are present in the region, which change the appearance of the surface. However, there are no dislocations outside of the channel region. In the case shown in Fig. 20, the width of the MCE layer (W) was 195 µm and the thickness (T) was 12 µm.57)
Fig. 20. Typical as-grown surface of GaAs MCE layer. A rough area appears in the central region, where a large number of dislocations have propagated through the microchannel.57)
Download figure:
Standard image High-resolution imageFigure 21 shows a cross sectional TEM image of as-grown MCE GaAs on silicon taken by Tamura of Joint Research Center for Atom Technology.53) It is clearly seen that threading dislocations are blocked by the SiO2 film deposited on the GaAs buffer layer, which was grown on a (001) silicon substrate by MOCVD. It is also seen that the dislocations continue to the epitaxial layer through the microchannel, which is 5 µm in width. Since the dislocations propagate on the {111} plane, the dislocated area is broadened as growth proceeds, as seen in the figure. Hence, to realize a dislocation-free area as large as possible, one should grow an MCE layer that is as wide as possible while keeping the thickness as small as possible.
Fig. 21. Cross-sectional TEM image of GaAs MCE on silicon substrate taken by Tamura (JRCAT).53)
Download figure:
Standard image High-resolution imageAn attempt to decrease the density of dislocation generated at a heteroepitaxy interface was reported by Tsauer et al. in 1982.58) They employed halogen CVD and grew GaAs from narrow windows cut in a SiO2 film deposited on a buffer layer of Ge on a silicon substrate. They obtained a dislocation density of less than 104/cm2 in the laterally grown area. At that time, since a value of 104/cm2 was not attractive for GaAs-on-silicon technology for optical use, which requires dislocation free GaAs for the fabrication of LDs, this work did not draw much attention. However, once it was found in 1989 that a dislocation-free layer can be obtained in a laterally grown area,54) this technology started to attract strong interest.
Usui et al. used MCE to grow GaN on sapphire to decrease the dislocation density in 1997,59) and Nakamura et al. employed this technology to fabricate a nitride LD and succeeded in obtaining a lifetime of more than 10,000 h under cw operation at 20 °C in 1997.60) Since then, MCE has been widely used as a method to reduce the dislocation density.
4. Low dimensional structures
4.1. Self-assembly
The proposal by Arakawa and Sakaki61) to use a low-dimensional structure (LDS) for a high-performance semiconductor laser stimulated the development of technology to fabricate wire and dot structures by crystal growth and also attempts to advance the growth science of LDSs. For the growth of a dot structure, a self-assembly process of InAs dots on a GaAs substrate was employed very successfully in 1985.62) In 1990, Sasaki's group tried to grow InxGa1−xAs (0 ≦ x ≦ 1) layers on InAs and InP substrates and found that the formation of dots occurred.63) These techniques utilize a self-stopping effect of the dot size induced by the strain between the dot and the substrate. Such self-assembled dots are already used in the production of quantum dot lasers, because the method gives high-density and high-quality dots. The production of quantum dot lasers is one of the major successes of crystal growth.
Regarding the science of crystal growth, the formation of dots or small islands after the growth of some layers on a lattice mismatched substrate has been studied for a long time. Such growth is known as the Stranski–Krastanov (SK) mode of heteroepitaxy. A great deal of work has been devoted to studying the growth of dots on various substrates. In this respect, the development of quantum dot devices has encouraged fundamental studies of SK growth.
4.2. Non-planar substrates
However, the self-assembly technique cannot be used for the fabrication of wire structures and dots on arbitrary substrates. The positioning of the dots is also a difficult problem. If we can solve these problems, the range of LDS applications will be greatly expanded.
One of the methods used to solve these problems is to perform growth on non-planar substrates. Kapon et al. employed a substrate with grooves and obtained GaAs/GaAlAs wire structures on the bottoms of the grooves.64,65) Fukui et al. used SAE and obtained a GaAs dot on the top of a GaAlAs truncated pyramid.66)
In developing the technology for growth on a non-planar surface, a very basic phenomenon in growth science has been found. Isu et al.67–71) and later Shen et al.72–76) studied the surface diffusion between facets. The macroscopic surface diffusion between facets, which is known as intersurface diffusion, plays an important role in determining the final facet that appears in the growth of an LDS. By utilizing the intersurface diffusion one can create an LDS and can control its shape.
Figure 22 shows growth pyramids of GaAs grown by MBE on mesas produced by photolithography and chemical etching.77) The figure shows a scanning electron microscopy (SEM) image taken in real time during the growth. The growth was conducted by a micro-probe RHEED/SEM MBE system, which is schematically shown in Fig. 23. The micro-probe RHEED/SEM MBE system is very powerful for studying the growth of LDSs. This apparatus was developed by Ichikawa et al.78) and others.67,68,79–81)
Fig. 22. Real-time image of growing pyramids. First, mesas are formed on a GaAs(111) B substrate by photolithography and chemical etching, then the growth is conducted in an MBE system equipped with a high-resolution scanning electron microscope (see Fig. 23).77)
Download figure:
Standard image High-resolution imageFig. 23. Micro-probe RHEED/SEM MBE system. The electron gun of a high-resolution scanning electron microscope is installed on the top of the custom-made MBE system.
Download figure:
Standard image High-resolution imageIt was found that in the MBE of GaAs the direction of intersurface diffusion is reversed by changing the arsenic pressure. Figure 24 schematically represents the growth rate distribution on a (001) facet adjacent to a (111) B facet when intersurface diffusion occurs. Here, gallium adatoms diffuse from the (111) B facet to the (001) facet under a flux of As4. Gallium adatoms diffusing from (111) B facet are incorporated one by one at the steps generated by two-dimensional nuclei on the (001) facet.
Fig. 24. Definitions of RCORNER, RPLANAR, and G. If RCORNER is larger than RPLANAR, the Ga flows from the side to the top surfaces.
Download figure:
Standard image High-resolution imageThus, the intensity of the lateral flux of gallium decreases and the local growth rate decreases as the distance increases from the boundary between the two facets. The decay of the growth rate follows an exponential law, and from the slope in a logarithmic plot one can obtain the incorporation diffusion length λinc, which is of µm order. We define G as the ratio of RCORNER to RPLANAR, as shown in the figure, where RCORNER and RPLANAR are the growth rates at the boundary on the (001) facet and infinitely far from the boundary, respectively. A value of G of larger than unity means that intersurface diffusion occurs from (111) B to the (001) facet, while G of less than unity indicates that the flow occurs in the opposite direction.
Figure 25 gives the change in G as the As4 pressure is increased.82) There are three arsenic pressure regions in which intersurface diffusion occurs in a certain direction. In regions 1 and 3, gallium adatoms diffuse from (111) B facet to the (001) facet, and in region 2 the direction is reversed. By making use of the inversion of the surface flow, one can control the shape of the growth pyramids, such as those shown in Fig. 22. During the growth, truncated pyramids with (111) B tops and {110} sides on a (111) B GaAs substrate are obtained, and as the growth proceeds the tops finally become sharp and true pyramids are formed. However, if the arsenic pressure is further increased, the direction of the gallium flow is changed and the tops again become flat, as shown in Fig. 26, similarly to in region 3 of Fig. 25. On the other hand, if the arsenic pressure is decreased, the flat tops again change to sharp tops as shown in Fig. 26.77) As seen in the figure, the truncated pyramids grown on a (111) B substrate show a reversible change in the top shape, and this phenomenon can be utilized to fabricate dot structures as explained by Fukui et al.66)
Fig. 25. G as a function of As4 pressure. As the As4 pressure is increased, the flow direction is reversed twice.82)
Download figure:
Standard image High-resolution imageFig. 26. Real-time control of pyramidal growth.77) The arsenic pressure was kept at 0.7 × 10−3 Pa until 360 min (a), then it was changed to 1.5 × 10−3 Pa until 420 min (b), and then changed to its original value. After 60 min, the top became sharp again (c).
Download figure:
Standard image High-resolution image5. Future aspects
As we have seen in the history of crystal growth, the art and science of crystal growth have had strong impacts on industry and hence on modern human society. We can classify the impacts into two kinds. The first kind of impact was when new materials such as quartz, ruby/sapphire, Ge, Si, GaAs, GaN, and etc. were crystallized. The second was when new techniques of crystal growth such as the Czochralski, floating zone, halogen vapor epitaxy, LPE, MBE, MOCVD, and HM2 techniques were developed. In future, new impacts on society will result from the discovery of new materials and the invention of new growth technologies.
Regarding the new materials to be grown, there are many possibilities. Among them, the most important will be the growth of diamond. Diamond has already been grown by a temperature gradient method under a high pressure and high temperature83) and by microwave-plasma-assisted CVD.84) Single crystals of more than 10 mm in diameter have been grown by both techniques, although this size is insufficient for use in the electronics industry. However, if large bulk crystals of diamond become available, they will have many applications in high-power and high-frequency electronic devices. Diamond is one of the most important materials and is greatly required to develop the growth technology for producing larger bulk crystals.
As discussed earlier, GaN and its alloy grown on sapphire by two-step growth gave blue light to the world. Nitride semiconductors are also extremely important materials for future electronic and optical devices. On the other hand, since large bulk crystals of GaN cannot yet be realized, one should use a substrate of another material such as sapphire, which causes many problems such as defect and strain generation at the heteroepitaxy interface. It is well known that nitride electronic devices such as diodes and transistors show much better performances at higher power and higher frequency than those of silicon. Hence, once a large bulk crystal of GaN is grown, the range of application of nitride-based electronics will be greatly expanded. There have been many attempts to grow bulk crystals of GaN, such as by high-pressure and high-temperature solution growth,85) halogen CVD, the ammonothermal method,86,87) and Na flux LPE.88,89) However, there is some distance between present results and the desired size and quality of bulk crystals.
The growth of bulk SiC also has a long history, although it is still in the development phase. In its history, a great amount of efforts have been made to control the polytypes and to decrease the number of micropipes, which are composed of screw dislocations. To control the polytypes, the use of a vicinal substrate of the required polytype was found very effective by Matsunami's group.90,91) By keeping the supersaturation low on the vicinal surface, one can grow SiC in step-flow mode, which prevents the generation of two-dimensional nuclei that give rise to the growth of polytypes other than that of the substrate.
The decrease in the micropipe density in sublimation growth is realized as a result of decreasing the dislocation density by choosing better growth conditions, especially the temperature uniformity, which is not so easy because the growth temperature is extremely high such as 2200 °C. The micropipe density depends on the size of the grown crystal, but wafers of as large as 6 in. with a density of a few micropipes per cm2 are now commercially available as well as micropipe-free wafers of 4 in. However, the densities of dislocations and other defects are still high and further technological development is required.
There are a lot of materials in addition to semiconductors and oxides that are required to be grown as single crystals. For such crystallization, one has to apply existing technologies or to develop new methods for crystal growth. There is plenty of room for the next generation of scientists to meet this challenge in future.
6. Conclusions
Looking back at the history of modern society, it is clear that developments in the art and science of crystal growth have had huge impacts on industry, through which drastic changes in human life have occurred. The growth of high-purity and perfect crystals of germanium made it possible to invent the transistor. The invention of the transistor had a huge effect on human life through the successive developments in silicon technology, which is also based on silicon crystal growth.
We are now benefiting from well-developed information society based on silicon integrated circuits, which are installed in all kinds of computers, cell phones, automobiles, aircraft, ships, and so forth. Technological developments in compound semiconductor crystal growth have also impacted society through the rapid increase in the rate of information transmission through the use of semiconductor lasers, which were realized by the invention of LPE.
Another huge impact resulted from the invention of two-step growth in the heteroepitaxy of highly-lattice-mismatched systems (HM2). The successful growth of a p–n junction of a nitride semiconductor by this technique made it possible to fabricate UV–blue and white LEDs and LDS. This has been an extremely successful development because nitride LEDs save enormous amount of energy and have contributed greatly to the realization of a less energy-intensive society. Thus, the Nobel Prize in Physics in 2014 was awarded for the invention of the p–n junction blue LED.
The art and science of crystal growth have contributed to an advanced modern society, and its further development is expected to be truly beneficial for society. For this purpose, the understanding of the art is strongly required. The art of crystal growth should be deeply based on science of crystal growth.
Biographies

Tatau Nishinaga received BS, MS, and Dr. degrees in Electronics from Nagoya University respectively in 1962, 1964, and 1967. He was an associate professor of Nagoya University (1970–1977) and a professor of Toyohashi University of Technology (1977–1983). In 1983 he became a professor of The University of Tokyo at the Department of Electronic Engineering. Since 2000, he was a professor of Meijo University in Nagoya and from 2002 to 2008 he became a president of Toyohashi University of Technology. Meanwhile, he was a visiting scientist of Institute of Crystallography, Academy of Science, USSR (1981–1982). He served as a president of the Japanese Association for Crystal Growth (JACG, 1994–1996), a president of the Japan Society of Microgravity Application (JASMA, 1996–1998) and a president of the International Organization for Crystal Growth (IOCG, 1995–2001). He received The Yamazaki-Teiichi Prize (2002), IOCG Laudise Prize (2004), and Outstanding Achievement Award of the Japan Society of Applied Physics (2012). He was awarded the title of professor emeritus from The University of Tokyo (2000) and Toyohashi University of Technology (2008).



























