Abstract
We have characterized mechanisms to improve the adhesion between printed electrodes prepared from silver nanoparticle inks and underlying polymer layers. Adhesion strength was significantly improved by sintering the inks above the glass transition temperature of the polymer underlayers, whereby enhanced adhesion was realized through interfacial fusion between the silver electrode layer and the underlying polymer layer. The surface energy of the underlayer was found to be an important factor in the improvement of adhesive strength, in that larger and thicker interfused regions between layers were observed for underlayers that had higher surface energies.
Export citation and abstract BibTeX RIS
Introduction
Compared to conventional vacuum deposition and subtractive pattering using photolithography, thin-film electronic device fabrication based on printing technology is expected to provide benefits such as more efficient material usage, low-temperature processing, and high productivity [1–7]. With printing, conductive inks and pastes are very important materials for realizing high performance electronic devices and circuits on plastic film substrates. Among the printable conductive materials, silver nanoparticle inks have become one of the most promising alternatives [8–18]. Since the sintering temperature of silver nanoparticles has decreased due to reductions in nanoparticle size, high electrical conductivities can be achieved at relatively low temperatures (<150 °C) [19–21]. This is of great advantage to the fabrication process of novel electronic devices such as flexible/wearable sensors that are compatible with the human body [22–24]. Plastic films such as PEN or PET are flexible substrate materials with low glass transition points of 150 °C and 90 °C, respectively. Silver pastes, the material typically used for printed electrodes, have not been applied for printed devices on plastic because they require higher sintering temperatures (>240 °C) to exhibit high conductivities [25–27]. Thus, silver nanoparticle inks are recognized as being the most suitable material for conductive layers in printed, flexible devices.
In flexible device applications, the adhesion strength of printed electrode layers is very important to achieve high durability against mechanical stress causing film deformation. Although silver electrodes prepared with nanoparticle inks have a major advantage in sintering temperature, their adhesion and mechanical stability require further improvements [28, 29]. Conventional silver pastes show relatively strong adhesion because the pastes contain a polymeric binder with some substituent groups that promote adhesion with underlayers [30–32]. In addition, mechanical durability can be further improved by using the cross-linkable binder polymers in silver pastes. In contrast, silver nanoparticle inks generally contain little or no binder polymers in order to maximize conductivity. Therefore, improving the adhesion of silver nanoparticle ink layers is a key issue in the research and development of printed electronics technology [33–35].
To address this issue, we recently reported on methods for improving the adhesion of printed silver electrodes prepared using nanoparticle inks [36]. In this research, we found that the sintering of silver electrodes above the glass transition temperature of the underlying polymer layers provides for an increase in adhesion by forming an interfused layer between the silver layer and the underlying polymer layer material. By using this technique, the mechanical durability against bending stress in organic thin-film transistor devices was dramatically improved due to the improvements in silver electrode adhesion that were achieved. In addition, other research groups have also reported on the adhesion of silver nanoparticle layers [29, 35, 37, 38]; however, the mechanisms for improving adhesion are not yet clear. A better understanding of the interfacial conditions between the silver nanoparticle layer and underlying polymer layer is very important to achieve good adhesion.
In order to investigate the improvement mechanisms for adhesion between silver electrode layers prepared by nanoparticle ink and polymer underlayers, we evaluated the adhesion strength of silver layers as a function of sintering temperature. Cross-sectional images of the interface between the silver electrode and underlying polymer were observed by using SEM and TEM. The mechanisms for improving adhesion strength are discussed by focusing on the glass transition temperature (Tg) and the surface energy of polymer underlayers.
Experiment
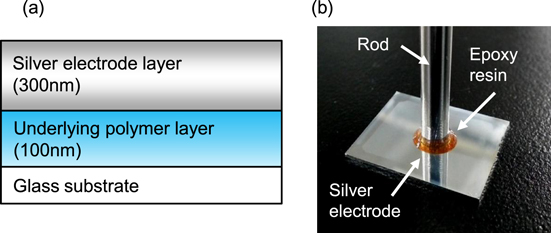
Poly(4-vinyl-phenol) (PVP; Sigma Aldrich, 436 224), Poly(methyl methacrylate) (PMMA; Sigma Aldrich, 370 037), and Teflon® (DuPont, AF1600X) were used as underlying polymer layers. A 5 wt% solution of PVP in 1-methoxy-2-propyl acetate (Kanto Chemicals, 01 948–00), a 5 wt% solution of PMMA in toluene (Kanto Chemicals, 40 500–05), or a 3 wt% solution of Teflon in Fluorinert™ (3M, FC-43) was spin-coated on a glass substrate and sintered at 150 °C for 1 h in ambient air to form a underlayer with a thickness of 100 nm (figure 1(a)). The surface of the Teflon layer was treated with weak oxygen plasma (10 W for 10 s) to improve wettability for the silver nanoparticle ink. Silver nanoparticles, which were dispersed in tetradecane with a silver concentration of approximately 55 wt% (Harima Chemicals, NPS-JL) was used as the printed silver electrode material. Since silver nanoparticle ink in this experiment consists of a silver nanoparticle with organic surfactant and a solvent (tetradecane), it does not contain any additive agents to improve the adhesion. The silver nanoparticle ink was spin-coated onto prepared substrates with each underlayer material. The thickness of silver nanoparticle layer before sintering was about 360 nm. The silver nanoparticle layers were then sintered at various temperatures (from 120 °C to 180 °C) for 1 h in ambient air, such that the thicknesses of silver layers were about 300 nm.
Figure 1. (a) A cross-sectional schematic of the silver electrode and underlying polymer layer. (b) Experimental set up image for the pull-off test.
Download figure:
Standard image High-resolution imageAdhesion strength between the sintered silver layers and the underlayers were measured by a pull-off method based on International Organization for Standardization (ISO) 4624. The pull-off method procedure is shown in figure 1(b) [39, 40]. A rod was fixed perpendicular to the sintered silver layer with an epoxy resin (#3459-MI, Mitsubishi Plastics, Inc.), which was also selected according to ISO 4624. The epoxy resin was annealed at 120 °C for 10 min. Next, the fixed rod was pulled in the perpendicular direction at a constant displacement rate of 120 mm s−1 using force gauge equipment (FGP-5, Nidec-Shinpo Co.) until the silver layer peeled off. The adhesion strength was calculated from the pulling strength (N) at which the silver layer peeled and its area.
The glass transition temperatures (Tg) for the underlayers were estimated by using differential scanning calorimetry (DSC) (TA instruments, Q2000), with rate of temperature increase and decrease of 10 °C min−1. The interfacial structure between the silver electrodes and underlayers were observed by using a scanning electron microscope (SEM) (JEOL, 7600-FE) at a 5 kV accelerating voltage, and transmission electron microscope (TEM) (JEOL, 2100-FE) at a 200 kV accelerating voltage. The measured sample for TEM analysis was fabricated using a focused ion beam system (JEOL 9360-FIB). The surface energy for the underlayers was calculated by the Owens–Wendt method using the contact angle of pure water and diiodomethane.
Results and discussion
Figure 2 shows silver electrode layer resistivity as a function of sintering temperature and SEM images of a silver layer sintered at 140 °C. As expected, the resistivity of silver electrode layers decreased with increased sintering temperatures. At sintering temperatures over 120 °C, the resistivity level was less than 10 μΩ cm, which is the same order as bulk silver. A minimum resistivity of 3.5 μΩ cm was obtained at a sintering temperature of 180 °C, which is approximately two times higher than the value for bulk silver (1.6 μΩ cm). It is thought that the difference in specific resistivity between the sintered silver electrode and the bulk silver is attributed to the thin-film structure. As shown in figure 2(b), a small void was observed in the sintered silver electrode. The resistivities for sintered silver electrode layers on each underlayer material showed the same tendency [41, 42]. The degree of sintering of silver nanoparticles also exhibited the same tendency on each underlayer material and the glass substrate, as shown in figure 2(b). From these results, we confirmed that the resistivity of silver electrodes was not affected by the underlayer material.
Figure 2. (a) Resistivity for the silver electrodes on underlayers as a function of sintering temperature. (b) SEM images of the surface morphology for silver electrodes sintered at 140 °C on each of the underlayers.
Download figure:
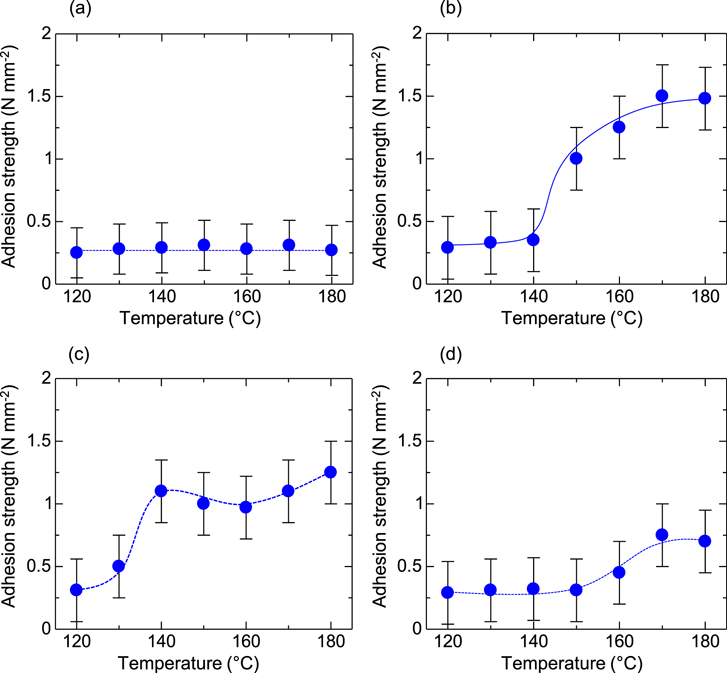
Standard image High-resolution imageFigure 3 shows the adhesion strength between silver electrode layers and each of the underlayers. The average values for ten samples were plotted and the dashed lines represent eye-guides of the plots. The adhesion strength on glass measured approximately 0.25 N mm−2 and did not change with the sintering temperature. In contrast, on several underlayers, the specific temperatures at which adhesion strengths improved were observed. The adhesion strength tended to increase gradually above these specific temperatures, and then became constant. The specific temperatures were 150 °C on PVP, 130 °C on PMMA, and 170 °C on Teflon. The maximum adhesion strengths were approximately 1.5 N mm−2 at 180 °C (on PVP), 1.3 N mm−2 at 180 °C (on PMMA), and 0.75 N mm−2 at 180 °C (on Teflon). The adhesion strengths on all underlayers after sintering below the specific temperatures showed almost same level of adhesion strength, ranging from 0.3 mm−2 to 0.4 N mm−2. Adhesion strength for a tape established using the standardized ISO 4624 as the reference for pull-off test measurements was 0.4 N mm−2 between the glass and itself. Thus, even for sintered silver electrodes on Teflon, which has a hydrophobic surface, practical values for the silver electrode adhesion strength were obtained.
Figure 3. Adhesion strength as a function of sintering temperature for the silver electrodes; (a) glass (b) PVP, (c) PMMA, and (d) Teflon.
Download figure:
Standard image High-resolution imageFigure 4 shows the relationship between the adhesion strength of a silver layer sintered at 180 °C and the surface energy of several underlayers. The surface energy of PVP, PMMA, and plasma-treated Teflon surfaces exhibited adhesion strengths of 44 mN m−1, 36 mN m−1, and 17 mN m−1, respectively. The adhesion strengths increased with increasing surface energy, which corresponds to the wettability of the surfaces. In this case, we have to consider the wettability of a silver nanoparticle with a polymer underlayer, because improvements in adhesion occurred through the sintering process, as shown in figure 3. From these results, we discovered that the adhesion strength of silver layers prepared with nanoparticle inks depends on the wettability of silver nanoparticles with the polymer underlayers. In fact, Joo et al mentioned the importance of surface energy on silver nanoparticle adhesion [27]. According to this report, surface energy of the silver nanoparticle affected adhesion. From above, certainly our results, which the adhesion strength of silver layers depends on the wettability of silver nanoparticles, are consistent with the literature-based tendency.
Figure 4. Relative adhesion strength versus surface energy of the underlayers.
Download figure:
Standard image High-resolution imageIn order to understand the origin of the specific temperature for each polymer, we investigated the correlation between the thermal properties and the dependence of sintering temperature on each polymer. Figure 5 shows the results of DSC measurement. The glass transition temperatures (Tgs) of each polymer were estimated from the changing point of heat flow. The estimated Tgs of PVP, PMMA, and Teflon were 140 °C, 130 °C, and 170 °C, respectively. This temperature is very close to the specific temperature to improve the adhesion strength on each polymer, as observed in figure 3. No clear peak was observed in the DSC measurement for the silver nanoparticle (figure 5(d)). From this result, we deduced that the mechanisms for adhesive strength between silver electrodes were improved by the intermixing between the silver layer and the underlayer at the interface by sintering at temperatures above Tg of the polymer material used as an underlayer.
Figure 5. DSC measurement of underlayers in powdery; (a) PVP, (b) PMMA, (c) Teflon, and (d) silver nanoparticles ink.
Download figure:
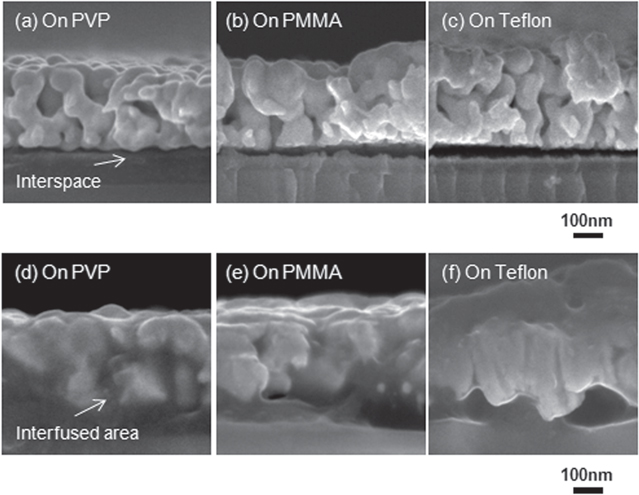
Standard image High-resolution imageTo confirm these improvement mechanisms, the interface between the silver electrode and underlayer was observed using an SEM. Figure 6 shows the cross-sectional images of the interface between the silver electrodes and each of the underlayers sintered at 120 °C and 180 °C. For a sintering temperature of 120 °C, the silver electrode adhered poorly to all underlayers and had several tens of nanometers of interfusion. The Tg for all polymers were higher than 120 °C, as shown in figures 6(a)–(c). In contrast, by sintering at 180 °C, a fused interface between the silver layer and all underlayers was observed. This indicates that the sintering of silver nanoparticles at temperatures above Tg for the underlayer promotes interfacial fusion and strong adhesion between the silver electrode layer and polymer underlayer [36]. In addition, the interfused area of silver and each polymer differed in figures 6(d)–(f). The silver layer interfused with the PVP underlayer over large areas; however, porous spaces were observed at the interface for PMMA and Teflon underlayers. We believe that the maximum adhesion strength for each underlayer depends on the interfused area of the silver layer. This is consistent with the relationship between the adhesion strength and surface energy, shown in figure 5.
Figure 6. Cross-sectional SEM images of the interface between silver electrode layers and underlayers sintered at (a)–(c) 120 °C and (d)–(f) 180 °C. There are interspaces in the interface between the silver electrode and underlayer sintered at 120 °C. Interfacial fusion occurred when sintered at 180 °C.
Download figure:
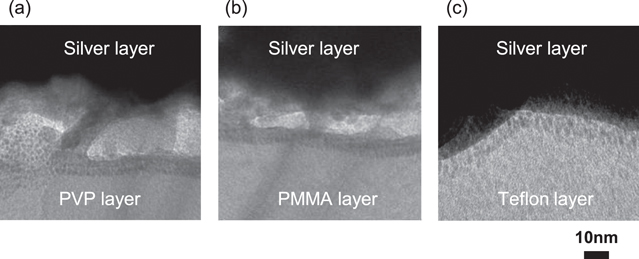
Standard image High-resolution imageIn order to analyze the interfused region in more detail, high-resolution cross-sectional TEM images were observed. Figure 7 shows the interface after sintering at 180 °C. The upper portion (black area) and bottom portion (white area) in the TEM images correspond to the silver layer and the polymer underlayer, respectively. The interfused layer consisted of metallic silver and polymer because a (111) diffraction pattern caused by the silver was observed [43]. The interfused layer was accompanied by nanoscale phase separation of the silver and polymer layers.
Figure 7. Cross-sectional TEM images of the interface between the silver electrode layers and underlayer materials; (a) PVP, (b) PMMA and (c) Teflon.
Download figure:
Standard image High-resolution imageIn addition, the thickness of the interfused layer on each underlayer differed. The interfused layer on PVP was clearly thicker than that on the PMMA and Teflon layers, whereby the thickness of the interfused layer on Teflon was approximately 15 nm. In addition to the area of the interfused region, we noted that the thickness of the interfused layer is also responsible for maximizing adhesion strength. Moreover, we observed that the thickness of interfused layer depends on the surface energy on each underlayer. It is thought that the silver nanoparticle layer is fluidized by the sintering process [44]. Therefore, the wettability of the silver nanoparticle layer to the underlayer is a very important factor for the penetration of silver into the underlayer. These results provide a consistent explanation for the adhesion improvement mechanisms for the silver nanoparticle layer.
Conclusions
In conclusion, we have demonstrated improvements in the mechanisms that promote adhesion between silver electrode layers prepared from printed nanoparticle inks and underlying polymer layers. Adhesion strength was increased significantly by sintering the silver electrodes at a temperature above Tg for the underlayer due to the formation of an interfused layer between silver and the polymer. The surface energy of the underlayer is a very important factor in achieving improved adhesion. Since the wettability of the underlayer was improved by using higher surface energies, larger and thicker interfused layers could be formed after sintering at temperatures above Tg.