Abstract
A facile, site-specific viral-templated assembly method was used to fabricate sensitive hydrogen sulfide (H2S) gas sensors at room temperature. A gold-binding M13 bacteriophage served to organize gold nanoparticles into linear arrays which were used as seeds for subsequent nanowire formation through electroless deposition. Nanowire widths and densities within the sensors were modified by electroless deposition time and phage concentration, respectively, to tune device resistance. Chemiresistive H2S gas sensors with superior room temperature sensing performance were produced with sensitivity of 654%/ppmv, theoretical lowest detection limit of 2 ppbv, and 70% recovery within 9 min for 0.025 ppmv. The role of the viral template and associated gold-binding peptide was elucidated by removing organics using a short O2 plasma treatment followed by an ethanol dip. The template and gold-binding peptide were crucial to electrical and sensor performance. Without surface organics, the resistance fell by several orders of magnitude, the sensitivity dropped by more than a factor of 100 to 6%/ppmv, the lower limit of detection increased, and no recovery was detected with dry air flow. Viral templates provide a novel, alternative fabrication route for highly sensitive, nanostructured H2S gas sensors.
Export citation and abstract BibTeX RIS
1. Introduction
Hydrogen sulfide (H2S) is a toxic gas released in petroleum, mining, paper, and water treatment industries [1] Although low concentrations of H2S, below 5 ppm, are innocuous, slightly higher concentrations, near 20 ppm, can cause eye and respiratory tract irritation. Furthermore, H2S concentrations at or above 100 ppm are considered immediately dangerous to life and health (IDLH), and may cause paralysis and even death [2, 3]. To minimize occupational health hazards, H2S gas levels must be monitored continuously. In particular, compact, low power consumption sensors with high sensitivity and low detection limit for personal exposure and mobile monitoring applications are highly desirable.
Nanostructured materials with high surface area-to-volume ratios are good candidates for chemiresistive gas sensors that address these needs. These materials facilitate interaction with analytes and support significant changes in electrical resistance due to analyte surface adsorption/desorption, with minimal power expenditure and a reduced device footprint. H2S gas sensors have been assembled from a variety of nanostructured metals and metal oxides [4–8]. Gold has received specific attention because its strong affinity for sulfur-containing compounds can be used to impart sensor selectivity [9–11]. Gold nanostructures, assembled with various methods, have been investigated both as sensitizers for other materials and as independent building blocks to fabricate H2S gas sensors. For example, for H2S detection, electrochemical deposition has been used to attach gold nanoparticles to carbon [12, 13] and polyaniline nanotubes [14]; sputtering has also been used to create discontinuous nanoscale gold films on carbon nanotubes [15] and 1-pyrenesulfonic acid-coated templates have been used to synthesize gold nanoparticles and nanowires on the surface of carbon nanotubes [16]. Additionally, gold-based H2S sensors have been assembled directly from thermally evaporated nanocrystalline gold films [17], thick chains of electrophoretically-assembled glycine-stabilized gold nanoparticles [4], and clusters of randomly deposited citrate-coated gold nanoparticles [5], Moreover, some of these gold-based and gold-functionalized devices report room temperature operation [4, 13, 14, 16] a condition which significantly reduces power consumption and is generally not feasible for metal-oxide-based H2S sensors.
Biological materials with nanoscale, hierarchical structures advantageous for gas sensing provide an alternative route to nanostructured material synthesis [18–21]. Fibrous matrices of eggshells [22] quasi-honeycomb structures of butterfly wings [23] and reticulated porous networks of wood [24] have been used to template solution-based synthesis of SnO2, α-Fe2O3, and ZnO, respectively. Not readily attainable with conventional fabrication or synthesis methods, these novel architectures with high surface area-to-volume ratios were found to be useful for chemiresistive H2S gas sensors at elevated operation temperatures following calcination to remove the biological template [22–24].
In this work, viral-templated gold nanowire H2S gas sensors were demonstrated. A gold-binding M13 filamentous bacteriophage, approximately 6–7 nm in diameter and 880 nm in length [25, 26], was used to template nanocrystalline gold. The high aspect ratio M13 virus has been used successfully to template numerous inorganic nanowires and nanowire assemblies, as well as for the fabrication of a range of device architectures [27–29]. These one-dimensional biological structures were well-suited for nanowire-based H2S gas sensor formation. Unlike previous bio-templated H2S sensors [22–24], the viral template was designed with specific affinity for the inorganic sensor material and the template was not removed prior to gas detection. The 2700 copies of gold-specific 8-mer peptide displayed on the length of the virus not only functioned as selective binding sites, but were also integral sensor components necessary for sensitive and effective H2S sensing at low ppb levels. To our knowledge, this is the first report of bio-templated, room temperature H2S gas sensors in which the template contributes more than simple geometry to sensing performance. These studies reveal the promise of biologically-directed synthesis for simple fabrication of highly sensitive, nanostructured gas sensors.
2. Experimental details
2.1. Assembly of viral-templated gold nanowire gas sensors
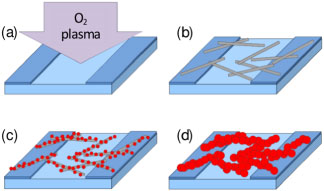
As depicted in figures 1(a)–(d), to form H2S gas sensors, bio-templated gold nanowires were assembled on electrodes fabricated on a Si/SiO2 substrate using a modification of a previously reported procedure [30, 31]. A gold-binding [26] M13 bacteriophage was used as the template. This particular clone displayed an 8-mer peptide (VSGSSPDS) with an affinity for gold on the N-terminus of each of 2700 copies of the pVIII major coat protein [26, 32]. The 300 nm thermal oxide layer electrically insulated the viral-templated nanowires from the underlying Si substrate. The Ti/Au (20 nm/180 nm) electrodes, which were 50 μm wide and separated by a 3 μm gap, were fabricated using standard photolithography, electron beam deposition, and lift-off techniques. Prior to nanowire assembly, the substrates with patterned electrodes were solvent cleaned with ultrasonication in acetone, isopropanol, and deionized water and activated with O2 plasma using a reactive ion etching (RIE, Surface Technology Systems) system at 100 W, 100 mT for 30 s. This plasma treatment was critical for uniform and non-specific adhesion of the viral template to the patterned substrates. The pre-patterned substrates were then incubated with gold-binding phage in tris-buffered saline (TBS, 50 mM Tris–HCl, 150 mM NaCl, pH 7.5) for 10 min. During this step, the gold-binding phages were adsorbed onto the substrate. The substrate was then washed and rinsed in TBS with 0.7% Tween 20 and deionized water. To control the density of phage on the substrate surface, and ultimately the number of parallel electrical connections between the electrodes, samples were made with three different phage concentrations: 1 × 108 pfu μl−1, 3 × 108 pfu μl−1, and 5 × 108 pfu μl−1. Gold nanoparticles were selectively bound to the gold-binding phage by submerging the substrate in a 5 nm diameter gold colloid solution of 5 × 1013 particles ml−1 (BBI Solutions.) for 1 h. The substrate was rinsed 3 times with deionized water and gently dried with air. The nanoparticles bound to the phages were used as seeds for electroless deposition of gold using Nanoprobes GoldEnhanceTM LM solutions. The electroless deposition time was varied from 3 to 12 min to control the nanoparticle size and electrical resistance of the gold nanowires formed. The viral-templated gold nanowire devices are hereafter referred to as 'as-assembled' devices. As-assembled devices were treated with O2 plasma for 30 s at 100 mT and 100 W using the RIE system, dipped in ethanol (Sigma-Aldrich) for 10 min, and gently blown dry with air. This two-step process removed the viral template and surface organics, as well as Au2O3 which may have been generated by exposure to O2 plasma [33, 34]. These devices will hereafter be referred to as 'ethanol-treated' devices and were used to evaluate the contribution of the viral template to device resistance and sensing performance.
Figure 1. Schematic representation of the sensor fabrication process for as-assembled devices. (a) O2 plasma treatment of pre-patterned gold electrodes to enhance surface hydrophilicity. (b) Non-specific adsorption of gold-binding phage on the patterned substrate. (c) Specific binding of 5 nm gold nanoparticles to the pVIII coat protein of the gold-binding phages. (d) Nanocrystalline gold nanowires formed through seeded, electroless deposition.
Download figure:
Standard image High-resolution image2.2. Morphological characterization
Scanning electron microscopy (SrEM, Phillips XL30 FEG) was used to determine the morphology and spatial distribution of the gold nanowires on the substrate. The number of seed particles per phage was quantified. A short electroless deposition of 1 min was used to slightly enlarge the gold nanoparticle seeds without merging them, making them easier to observe with SEM. The particles on 15 individual templates were counted for range and average. In addition, the areal fill factors of viral-templated gold nanowires on the Si/SiO2 substrate were determined for devices assembled with phage concentrations of 1 × 108 pfu μl−1, 3 × 108 pfu μl−1, and 5 × 108 pfu μl−1. For each concentration, a minimum of 10 locations were imaged and analyzed. Furthermore, the width of the gold nanocrystal components of the viral-templated nanowires was measured at various electroless deposition times. Approximately 100 gold nanoparticles were analyzed for each electroless deposition time. Transmission electron microscopy (TEM, Phillips Tecnai 12) was used to analyze the morphology and connection between the nanoparticles within the gold nanowires. For TEM sample preparation, gold nanowires were fabricated on unpatterned SiO2 substrates and dispersed in deionized water by ultrasonication. The dispersed nanowires were then loaded onto carbon-coated copper grids and dried in vacuum.
2.3. Resistance measurements
The room temperature resistance of each as-assembled viral-templated gold nanowire device was determined using two-terminal, current–voltage (I−V ) measurements in which the current was recorded as the applied voltage was swept from −0.3 to 0.3 V (Keithley 2636A sourcemeter) in 30 mV increments. The same procedure was used to measure device resistance after ethanol treatment.
2.4. Sensor performance analysis
Devices selected for gas sensor measurements were wire-bonded (West-bond Inc. 7499D) at room temperature to a copper printed circuit board (PCB) with 1% Si/Al wire before sensing analysis. Wire-bonded sensors were placed in a flow cell chamber with a gas inlet and outlet. A constant bias of 0.15 V was applied to each device and, after establishing a stable baseline resistance, sensing analysis was performed at ambient temperature and pressure under a constant flow rate of 200 sccm. The resistance change of each viral-templated gold nanowire sensor was measured with exposure to H2S gas. To vary analyte concentration, H2S gas was diluted using dry air as the carrier gas and each sensor was alternately exposed to H2S gas at the specified concentration and dry air for time intervals of 15 min and 30 min, respectively. A mass flow controller with LabView interface was used to control the H2S concentration and exposure time. A similar procedure was followed for selectivity analysis using NH3 and NO2 as the gas analytes.
3. Results and discussion
3.1. Morphological characteristics of nanocrystalline, viral-templated gold nanowires
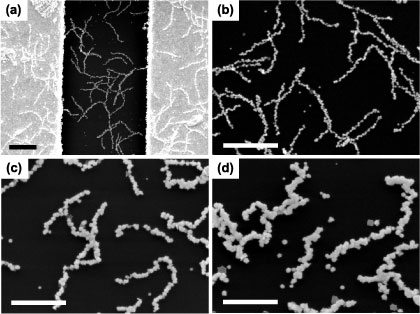
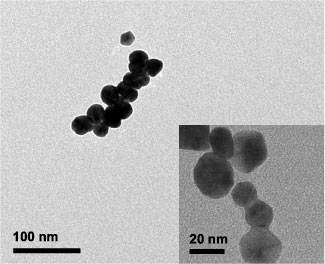
The morphology of the viral-templated gold nanowires which comprise the sensors was examined with SEM to study the effect of electroless deposition time and phage concentration on the as-assembled gold nanowires. A representative image of a sensor assembled with a phage concentration of 1 × 108 pfu μl−1 and an electroless deposition time of 3 min is shown in figure 2(a). Gold nanowires slightly less than 1 μm in length, composed of well-defined nanoparticles were seen randomly distributed on the substrate in addition to a few individual gold nanoparticles. The number of isolated nanoparticles was small in comparison to those incorporated in the nanowires. As shown in the high magnification SEM image in figure 2(b), the width and connectivity varied along individual nanowires, as well as from nanowire to nanowire. Further SEM analysis revealed that the number of gold nanoparticle seeds per template ranged from 31 to 61 with an average of 42. We, therefore, attributed the largest deviations in nanowire width and connectivity to differences in the density and arrangement of gold nanoparticle seeds along the gold-binding phage prior to electroless gold deposition. The size and morphological changes, which accompanied increased electroless deposition time, can be seen in figures 2(b)–(d). As the electroless deposition time increased from 3 to 12 min, the overall width and connectivity of the gold nanowires also increased. The average width of the resulting nanocrystals on nanowires for 3, 7, and 12 min of gold deposition were 29 ± 7 nm, 60 ± 13 nm, and 82 ± 16 nm, respectively. No difference in the morphology, structure, or distribution of nanowires on the substrate surface was observed with SEM after template removal by ethanol treatment as shown in the supplementary data (available at stacks.iop.org/Nano/25/135205/mmedia), figure S1. The TEM image in figure 3 revealed the detailed structure of the nanowires. Mostly shorter fragments (<200 nm) of nanowires were observed with TEM. This is likely because the fragile nanowires were fractured during sample preparation. The nanoparticles within the nanowires were polydisperse. Both single chain and multi-chain nanoparticle arrangements were observed. At higher magnifications near-point-contact connections between nanoparticles that, in many cases, structurally and electrically held the viral-templated gold nanowires together were observed.
Figure 2. Morphology of viral-templated gold nanowires within fabricated devices assembled with a 1 × 108 pfu μl−1 phage concentration and a range of electroless deposition times. (a) Low magnification SEM image that shows viral-templated gold nanowires with a 3 min electroless deposition time on 50 μm electrode and across 3 μm gap. Scale bar is 1 μm. High magnification SEM images of viral-templated gold nanowires with electroless deposition times of (b) 3 min, (c) 7 min, and (d) 12 min. Gold nanoparticles are assembled in a bead-like nanowire form with increasing nanowire widths corresponding to increasing electroless deposition time. Scale bars are 1 μm.
Download figure:
Standard image High-resolution imageFigure 3. Morphology and connectivity of viral-templated gold nanowires. TEM image shows a fragment of a viral-templated gold nanowire that was removed from the substrate with ultrasonication. Scale bar is 100 nm. High magnification TEM image (inset) shows fused, polydisperse gold nanoparticles within a different nanowire fragment. Scale bar is 20 nm.
Download figure:
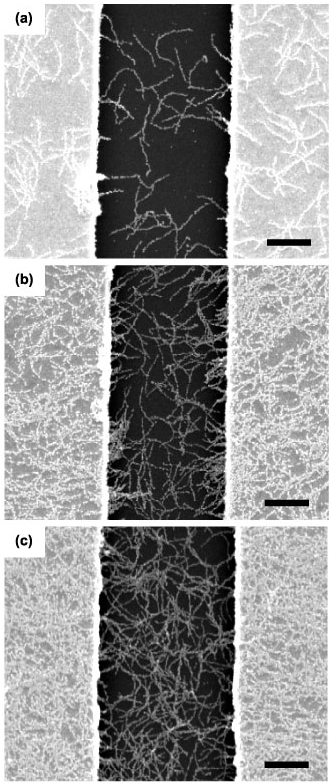
Standard image High-resolution imageFigures 4(a)–(c) show SEM images of the viral-templated gold nanowire sensors fabricated with increasing phage concentration and an electroless deposition time of 3 min. Phage concentrations of 1 × 108 pfu μl−1, 3 × 108 pfu μl−1, and 5 × 108 pfu μl−1 yielded devices with an average gold nanowire surface coverage of 16%, 39%, and 49%, respectively. At a phage concentration of 1 × 108 pfu μl−1 both isolated and small clusters of nanowires were observed within the 3 μm gap between the electrodes. At higher phage concentrations, 3 × 108 pfu μl−1 and 5 × 108 pfu μl−1, gold nanowires formed a continuous, mesh-like structure between the electrodes. Given the relative size of the gold nanowires and electrode gap, multiple nanowires were required to physically bridge the gap between the two electrodes. As a result, a lower density of complete, physical connections was observed for the 1 × 108 pfu μl−1 phage concentration, as compared to the 3 × 108 pfu μl−1 and 5 × 108 pfu μl−1 phage concentrations.
Figure 4. Dependence of gold nanowire surface coverage on phage template concentration. SEM images of gold nanowire devices assembled with phage concentrations (a) 1 × 108 pfu μl−1, (b) 3 × 108 pfu μl−1, (c) 5 × 108 pfu μl−1 and a 3 min electroless deposition time. Scale bars are 1 μm.
Download figure:
Standard image High-resolution image3.2. Electrical characteristics of viral-templated gold nanowire devices
The effects of electroless deposition time and phage concentration on sensor resistance were studied. Devices displayed Ohmic behavior within the −0.3 and 0.3 V two-terminal measurement range at room temperature for all conditions investigated. Viral-templated sensor resistance is shown as a function of gold deposition time in figure 5(a) along with the device yield for each condition. Device yield is defined as the ratio of devices with measurable resistance (less than 10 GΩ) to the total number of devices fabricated. The sensor resistance decreased as the electroless deposition time increased, which is consistent with a previous report [30]. As evidenced by SEM analysis, at longer times more gold was deposited on the nanoparticle seeds resulting in more continuous, thicker nanowires. We attribute the increase in percentage yield to the increased physical continuity of the nanowires. Furthermore, we ascribe the reduced device resistance to the increase in cross-sectional area of the nanowires, in addition to the enhanced continuity caused by deposition. At all deposition times, the device-to-device resistance varies by 1–4 orders of magnitude. The large range of resistances observed at a given deposition time is attributed to the previously mentioned variations in nanowire thickness and connectivity associated with differences in density and arrangement of gold nanoparticle seeds along the gold-binding phage.
Figure 5. Dependence of electrical behavior of gold nanowire devices on electroless deposition time and phage concentration. The device yield associated with each assembly condition is written above the relevant data. Median resistances of the devices with maximum and minimum values are shown as a function of (a) electroless deposition time and (b) phage concentration.
Download figure:
Standard image High-resolution imageAs expected, the phage concentration also influenced sensor resistance. The median resistances for devices assembled with phage concentrations of 1 × 108 pfu μl−1, 3 × 108 pfu μl−1, and 5 × 108 pfu μl−1 and an electroless deposition of 3 min is shown in figure 5(b), in addition to the device yield at each concentration. As the concentration of phage increased, the median device resistance decreased. As previously discussed, the morphology of the sensors varies greatly with phage concentration. A low concentration of phage (1 × 108 pfu μl−1) resulted in devices with nanowires that produced relatively few physical connections bridging the electrode gap and higher concentrations of phage (3 × 108 pfu μl−1 and 5 × 108 pfu μl−1) produced a fairly dense and continuous network of gold nanowires across the entire gap. As the gold nanowire density increased the number of available conductive pathways also increased, causing the overall sensor resistance to be reduced. Furthermore, the reduced yield at the low concentration was attributed the low probability of creating a physical connection between the two electrodes. To briefly evaluate the stability of the as-assembled devices the resistance was re-measured after storage under ambient conditions for 4–5 months. The resistance of all re-measured devices increased, most by no more than a few percent but a handful by as much as an order of magnitude. More studies are required to explore the cause of the resistance change and the source of device-to-device variations.
To better understand the impact of the phage template and surface organics on sensor resistance, two-terminal measurements were also performed on ethanol-treated devices. Resistance distributions of as-assembled and ethanol-treated sensors fabricated using a 3 × 108 pfu μl−1 phage concentration and 3 min electroless deposition are shown in figure 6. As previously discussed, a large distribution of resistances was observed in the as-assembled devices with the peak of the distribution between 108 and 109 Ω. Following ethanol treatment the distribution remained broad; however the peak resistance decreased to 10−100 Ω. Nonconductive organic ligands act as energy barriers to charge transport via electron hopping in metal nanoparticle films and chains, often resulting in highly resistive materials [35–37]. The large decrease in resistance observed in ethanol-treated sensors was attributed to the removal of organic components such as peptides and viral template from gold nanoparticle surfaces resulting in reduced nanoparticle-to-nanoparticle energy barriers and enhanced charge transport [37–39].
Figure 6. Histograms of resistances for as-assembled and ethanol-treated devices fabricated using a 3 × 108 pfu μl−1 phage concentration and 3 min electroless deposition.
Download figure:
Standard image High-resolution image3.3. Sensing performance of viral-templated gold nanowire devices
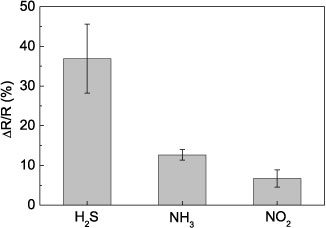
A characteristic real-time sensing response, defined as the change in resistance relative to the baseline resistance (ΔR/R0), and a calibration curve with respect to gas concentration for as-assembled gold nanowire sensors are represented in figures 7(a) and (c). These devices were assembled with a phage concentration of 3 × 108 pfu μl−1 and electroless deposition time of 3 min. After exposure to dry air flow for 5 h with constant bias applied, a stable resistance baseline was established for each device. However, within the first minute the resistance dropped sharply and then continued to slowly decline; by 10 min of dry air flow, all devices were stable with less than 5% change in resistance over time. Sensor resistance increased with exposure to H2S concentrations ranging from 0.025 to 0.5 ppm. This chemiresistive behavior was consistent with other reports of H2S sensors composed of gold NP chains [4, 5, 16], and films [17] in which charge transport between neighboring nanoparticles or nanocrystals is impeded by adsorption of H2S onto the gold surface causing resistance to increase. The sensitivity, defined as the slope of the linear region on the calibration curve, was 654%/ppm, within the linear range from 0 to 0.025 ppm. This sensitivity is more than an order of magnitude greater than other gold-based room temperature H2S sensors [4, 13, 17]. Saturation of sensor response was observed at concentrations above 0.025 ppm. The lowest detection limit, defined as the concentration at which the response is 3 times the signal-to-noise ratio, was 2 ppb. This value is lower than that achieved by H2S sensors composed of electrophoretically-assembled glycine-stabilized gold nanoparticles [4] and gold nanoparticles on 1-pyrenesulfonic acid-coated carbon nanotube templates [16], and comparable to the 3 ppb limit reported for sensors assembled from carbon nanotubes decorated with electrochemically deposited gold [13]. The response and recovery times, which are defined as the time to reach 95% of saturation resistance and the time for resistance to recover to 10% above the baseline resistance, were greater than 15 min and 30 min, respectively. Seventy percent recovery was observed within 30 min for all as-assembled devices, indicating desorption of the gas analytes from the surface. Faster recovery was observed with exposure to lower analyte concentrations such that devices exposed to 0.025 ppm H2S experienced up to 70% recovery within 9 min. The sensors exhibited some cross-sensitivity to NH3 and NO2, toxic gases which are also frequently present in water treatment and mining industries. Figure 8 shows the response of the devices to 0.5 ppm of H2S NH3 and NO2.
Figure 7. Representative room temperature H2S sensing behavior of (a) as-assembled and (b) ethanol- treated viral-templated gold nanowire devices. Sensors were alternately exposed to H2S and dry air for intervals of 15 and 30 min, respectively. Dashed line indicates the H2S concentration to which the sensors were exposed at each time. Calibration curve of (c) as-assembled and (d) ethanol-treated sensors showing sensor response to H2S concentrations between 0 and 0.5 ppm. Inset in (d) shows calibration curve of ethanol-treated devices for H2S concentrations between 0 and 40 ppm.
Download figure:
Standard image High-resolution imageFigure 8. Sensing response of viral-templated gold nanowire sensors to exposure to 0.5 ppm of H2S, NH3, and NO2 gases.
Download figure:
Standard image High-resolution imageTo highlight the importance of the gold-binding peptides and viral template on device behavior, the sensing performance of ethanol-treated devices, in which organics were removed, was also analyzed. A characteristic real-time sensing response and a calibration curve for ethanol-treated gold nanowire sensors are shown in figures 7(b) and (d). Ethanol-treated devices increased in resistance with exposure to H2S concentrations ranging from 0.025 to 40 ppm. These sensors maintained a linear response up to 0.5 ppm with a sensitivity of 6%/ppm, and a 6 ppb lower limit of detection. Like the as-assembled sensors, response times were greater than 15 min; however, a considerably slower initial response rate was observed for the ethanol-treated than for the as-assembled devices. No recovery was observed in these sensors. This irreversible behavior suggests the continued presence of analyte on the gold nanowire sensor surface. Similar behaviors have been reported at room temperature for H2S sensors based on ligand-free gold thin films [17] and citrate-coated gold nanoparticles [5, 16], due to strong Au–S affinity. Recovery was only attained in these devices at temperatures >140 °C.
The sensing behaviors of as-assembled and ethanol-treated sensors were notably different. As-assembled devices, in which the viral template and gold-binding peptides were intact, exhibited a slightly decreased lower detection limit (3×), decreased dynamic range (20×), and a substantial sensitivity increase (100×) compared to ethanol-treated devices. Moreover, the analyte adsorption and desorption rates of the as-assembled sensors were markedly faster than the ethanol-treated sensors. This behavior suggests that, although the viral template with the gold-binding peptide was intended primarily for structural assembly of nanowires, it also played an active role in device–analyte interaction.
Biological molecules incorporate a range of chemical moieties which enable extraordinary diversity and specificity for in vivo processes. These same attributes have proven useful in discrete sensor and electronic nose applications. Biological molecules including proteins [40, 41], peptides [42–44], antibodies [45], and DNA [46] have been successfully integrated into gas or vapor phase sensors to impart analyte specificity. Of particular relevance to these studies is the use of peptides. For example, piezoelectric-based sensors have utilized molecular modeling in conjunction with oligopeptide mimics of human olfactory [41] and dioxin [42] receptors to detect vapor phase acetic acid [41], ammonia [41], and dioxin [42]. The same oligopeptides have also been used to sensitize silicon nanowire-based chemiresistive sensors [44]. Furthermore, peptides identified with a combinatorial phage display library [47], in a process very similar to that used to select the gold-binding peptide found in these studies, have been used for detection of trinitrotoluene (TNT) with both fluorescence quenching [48] and conductance-based field effect transistor (FET) [49] device platforms. In the former, the entire virus with high copy peptide fusions was incorporated into the device [48]. Indirect evidence exists that, indeed, the gold-binding peptide may have an affinity for sulfur found in H2S gas. Specifically, the pVIII major coat protein of the unmodified or wild-type M13 virus has been recently reported to display an affinity for sulfur [50]. The carboxyl groups associated with acidic amino acids such as aspartate (D) and glutamate (E) found within the wild-type pVIII coat donate electrons to sulfur, creating S–O and C–S bonds [50]. Like the wild-type M13, the gold-binding phage template displays acidic amino acid (aspartate, D) on its pVIII coat and may, therefore, share this attraction to the H2S analyte, potentially increasing device sensitivity and response rate. In addition, amine groups have demonstrated an affinity for H2S [51–53]. Each of 2700 copies of the pVIII protein found along the length of the viral template is terminated with an amine group, which may again cause increased sensitivity and enhanced response rate. Moreover, very rapid initial response rates were observed in H2S sensors fabricated from glycine-stabilized gold nanoparticles on which amine groups were also displayed [4].
Alternatively, the gold-binding peptide fusion and/or phage template may interact with the H2S analyte or its decomposed, adsorbed products without exhibiting a specific affinity. The gold-binding phage template displays an 8-mer peptide known to preferentially bind to and reduce gold in solution [26]. Specifically, hydroxyl-containing amino acids such as serine (S) can act as anchoring sites for peptide adsorption to Au surfaces [26, 54, 55]. The presence of the high affinity gold-binding peptide, rich in serine (S), on the surface of the as-assembled devices, may sterically hinder or decrease available adsorption sites and weaken analyte binding to the gold surface resulting in reduced dynamic range and enabling device recovery. As described here, a few potential mechanisms, which may act alone or in combination, could account for the active role of the viral template within the H2S sensor. Yet, further studies are necessary to fully understand the function of the viral template and gold-binding peptides in sensor performance, as well as in adsorption and desorption kinetics.
4. Conclusion
We have demonstrated viral-directed assembly of very sensitive, nanocrystalline gold H2S gas sensors which operate at room temperature. The M13 bacteriophage template enabled facile control over device morphology, creating discrete nanowires from chains of gold nanoparticles. Electroless deposition time and phage concentration were used to adjust individual nanowire width and connectivity, as well as to manipulate the nanowire surface coverage of the device. Increased nanowire width, connectivity, and surface coverage decreased sensor resistance. As-assembled viral-templated gold nanowire sensors with template and binding peptides intact, exhibited high sensitivity near 650%/ppm, a very low limit of detection of 2 ppb, and 70% recovery within 9 min for 0.025 ppm H2S. Upon removal of the viral template and binding peptides using O2 plasma treatment and an ethanol dip, sensor resistance dropped by several orders of magnitude, the limit of detection increased, and sensitivity fell by more than a factor of 100. Furthermore, the initial rate of sensor response to H2S exposure was substantially reduced and recovery was lost. The presence of the bacteriophage template and gold-binding peptide clearly plays a sizable role in device resistance and is critical to H2S sensing. Bio-templated materials not only have the potential to generate high surface area-to-volume nanostructured architectures desirable for gas sensing, but may exhibit additional functionality which facilitates and even enhances device performance.
Acknowledgments
The authors will like to thank E L Hu (Harvard University) and A M Belcher (MIT) for the gift of the gold-binding phage (p8#9). We are also grateful to Myung Group members Heng Chia (Charles) Su and Nicha Chartuprayoon for their helpful discussions and assistance with the gas sensor measurement system. These studies made use of the Central Facility for Advanced Microscopy and Microanalysis (CFAMM) and Center for Nanoscale Science and Engineering (CNSE) at UCR.