Abstract
A one-step reduction/functionalization strategy for the synthesis of carboxylate-functionalized graphene nanosheets is reported in this paper. 1-pyrenecarboxaldehyde (PCA) is introduced as a new reductant for the chemical reduction of graphene oxide (GO), serving three roles: reducing GO to graphene nanosheets (GNs), stabilizing the as-prepared GNs due to the electrostatic repulsion of the oxidation products of PCA (1-pyrenecarboxylate, PC−) on the surface of the GNs and anchoring Pt nanoparticles (Pt NPs) with high dispersion and small particle size. Transmission electron microscopy shows that Pt NPs with an average diameter of 1.3 ± 0.2 nm are uniformly dispersed on the surface of the PC−-functionalized GNs (PC−-GNs). The obtained Pt NPs/PC−-GNs nanohybrids have higher electrocatalytic activity and stability towards methanol oxidation in comparison with Pt NPs supported on GNs obtained by the chemical reduction of GO with the typical reductant, hydrazine.
Export citation and abstract BibTeX RIS
1. Introduction
Due to their unique electronic, mechanical, and thermal properties, two-dimensional graphene nanosheets (GNs) and graphene-based materials have received significant attention in a variety of fields, including nanoelectronics, capacitors, fuel cells and chemo/biosensors, since they were first reported in 2004 [1–14]. Various methods have been developed to produce GNs, such as micromechanical cleavage, epitaxial growth, chemical vapor deposition, direct liquid exfoliation and solution-based chemical reduction of exfoliated graphene oxide (GO) [1, 15–19]. Among these methods, chemical reduction of exfoliated GO with a typical reductant, such as hydrazine or its derivatives, has been used widely due to the advantages of large-scale and low-cost production for GNs. However, precaution must be taken when large amounts of highly toxic hydrazine are used. On the other hand, due to their hydrophobic nature and strong π–π stacking tendency, GNs obtained from the chemical reduction of GO generally suffer from limited solubility, or even irreversible agglomeration in water, resulting in severe difficulty in further processing and application. Thus, it is particularly important to prevent the aggregation of GNs, because most of their unique properties are associated only with individual layers or few layers of nanosheets [20]. Recently, various stabilizers, such as lignin and cellulose derivatives, polymeric surfactants, DNA, cyclodextrin, and amino acid, have been reported to improve the dispersibility of GNs in aqueous solution [21–25].
On the other hand, it has been reported that noble metal nanoparticles (NPs)/GNs nanohybrids have excellent performance in heterocatalysis, chemo/biosensors and fuel cells [10–14, 26–32]. In these cases, a high dispersion of noble metal NPs on the surface of GNs is usually desirable. To achieve this aim, it is necessary for GNs to introduce more functional groups, such as carboxyl, to anchor the precursors of noble metal ions or noble metal NPs. Recently, carboxyl-functionalized GNs were prepared by partial reduction of GO and exhibited good solubility in water [33]. However, partial reduction of GO will result in large structural defects, which will lower the overall strength, electronic conductivity and corrosion resistance of the obtained GNs. With the view of most effectively utilizing the remarkable properties of GNs, it is desirable that the GO should be reduced as completely as possible. But the higher the reduced extent of GO, the lower the amount of functional groups remaining. Also, the obtained GNs will easily aggregate, resulting in the loss of the unique properties of GNs. Moreover, there are insufficient binding sites to anchor the precursors of noble metal ions or noble metal NPs due to the limited carboxyl groups remaining on the edge of the GNs. Can we overcome this contradiction to prepare highly reduced GNs with abundant carboxyl groups, only using a reductant?
Herein, we report an alternative strategy to synthesize carboxylate-functionalized graphene nanosheets for high dispersion of platinum nanoparticles: reduction of graphene oxide via 1-pyrenecarboxaldehyde (PCA) (scheme 1). PCA is a bifunctional molecule containing a pyrene moiety and one aldehyde group. Many PCA molecules can be adsorbed on the surface of GO via strong π–π stacking and hydrophobic interactions between the pyrene moiety and the basal plane of graphite [34, 35]. The aldehyde group of PCA acts as the reductant for the chemical reduction of GO, and is simultaneously oxidized to a carboxylate group under alkaline conditions. This results in highly reduced GNs with a large number of carboxylate groups on their surfaces (PC−-GNs). The large number of negatively charged carboxylate groups not only prevents the reduced GNs from aggregation in water by electrostatic repulsion, but also endows the reduced GNs with a uniform distribution of binding sites to anchor and grow metal nanoparticles. Due to their many potential applications, ranging from advanced sensors to highly efficient fuel cells [26–32], Pt NPs were selected as the model, and the dispersion of Pt NPs on the as-prepared carboxylate-functionalized GNs has been performed. The results show that Pt NPs are dispersed uniformly on the surface of the PC−-GNs and the obtained Pt NPs/PC−-GNs nanohybrids have superb performance for the electro-oxidation of methanol.
Scheme 1. Schematic diagram for the reduction of GO with different reductants and the dispersion of Pt NPs on GNs.
Download figure:
Standard image High-resolution image2. Experimental details
2.1. Materials
Graphite powder (SP-1 grade, 325 mesh) and 1-pyrenecarboxaldehyde (PCA, 99%) were purchased from Alfa Aesar. Inc. Ultra-pure nitrogen was used for the de-aeration of the electrolyte solution. Other chemicals were of analytical grade and used as received.
2.2. Preparation of PC−-GNs and GNs
GO was synthesized from graphite powder (spectral purity) by a modified Hummers method [36, 37]. The procedure for preparation of PC−-GNs was as follows: GO (40 mg) was dispersed in N,N-dimethylformamide (DMF, 160 ml) under ultrasonication (240 W) for 1.5 h. Under a N2 atmosphere, PCA (575 mg) as reductant was added into the GO suspension with stirring for 30 min. After NaOH solution (1.0 M, 2.0 ml) was added dropwise, the solution was heated to 100 ° C with continuous stirring for 20 h. The resulting suspension was then filtrated and washed with copious ethanol and double-distilled water. The obtained product, denoted as PC−-GNs, was dried at 50 ° C in vacuum for 12 h. For comparison, GNs were prepared under the same procedure as described above, where hydrazine replaced PCA and was used as the reductant.
2.3. Preparation of Pt NPs/PC−-GNs and Pt NPs/GNs nanohybrids
Deposition of Pt NPs on the PC−-GNs (or GNs) was carried out in aqueous solution. The details are as follows: PC−-GNs or GNs (16 mg) was mixed with H2PtCl6 (19.3 mM, 1.06 ml) in double-distilled water (15 ml) under ultrasonication for 30 min. Then, fresh NaBH4 solution (52.6 mM, 5 ml) was added dropwise with continuous stirring for 60 min. The products (denoted as Pt NPs/PC−-GNs or Pt NPs/GNs) were centrifuged and washed three times with double-distilled water, then dried in a vacuum oven at 60 ° C for 12 h.
2.4. Characterization
Fourier transform infrared spectra (FT-IR) were obtained from a Fourier transform infrared spectrometer (Nicolet, 6700). Thermogravimetric analysis (TGA) was performed on a NETZSCH STA 409PC at a heating rate of 10 °C min−1 in a N2 atmosphere. The x-ray diffraction (XRD) patterns were obtained on a D8 Advance (Bruker) x-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). Atomic force microscope (AFM) images were collected using an AFM (Multimode, Veeco Instruments Inc). X-ray photoelectron spectroscopy (XPS) analysis was carried out on a K-Alpha 1063 x-ray photoelectron spectrometer (Thermo Fisher Scientific). All the obtained spectra of XPS were calibrated to a C 1s peak at 284.0 eV. Raman spectra were recorded on a Raman spectrometer (Labram-010, England) using a 633 nm He–Ne laser beam. Transmission electron microscope (TEM) images were obtained using a TECNAI G2 high-resolution TEM operating at 200 kV. The Pt loading mass for the Pt NPs/PC−-GNs (or Pt NPs/GNs) catalyst was determined by inductively coupled plasma–atom emission spectroscopy (ICP-AES, Spectro Ciros) and the results are shown in table S1 (available at stacks.iop.org/Nano/24/395604/mmedia).
2.5. Electrocatalytic study
For electrochemical investigations, a glassy carbon (GC, 5 mm diameter) electrode was polished with slurries of 0.5 and 0.03 μm alumina successively and washed ultrasonically in double-distilled water prior to use. The catalyst ink was prepared by dispersing the catalyst (4 mg) in water (2 ml) by sonication. When a dark homogeneous dispersion was formed, 20 μl of the ink was dropped onto the GC electrode using a micro-syringe. After drying in air, the electrode was coated with 5 μl of 0.05 wt% Nafion ethanol solution. All electrochemical measurements were performed on a CHI660B electrochemical workstation (Chenhua Instrument Company of Shanghai, China). A conventional three-electrode glass cell was used with a platinum wire as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. All the potentials reported in this paper were with respect to SCE. Double-distilled water was used throughout.
3. Results and discussion
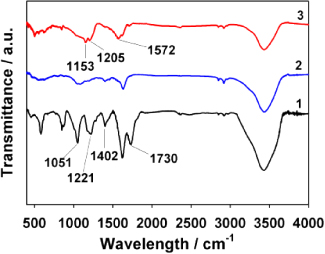
The reduction of the oxygen-containing groups in GO by PCA is confirmed by FT-IR spectroscopy (figure 1). The FT-IR spectrum of GO (curve 1) shows some characteristic peaks corresponding to the oxygen functionalities, such as the C=O stretching vibration peak at 1730 cm−1, the O–H deformation vibration peak at 1402 cm−1, the C–O (epoxy) stretching vibration peak at 1221 cm−1, and the C–O (alkoxy) stretching peak at 1051 cm−1 [38]. As shown in curve 2 and 3, after 20 h reduction of GO with hydrazine or PCA, the intensities of these characteristic peaks decrease dramatically and some of them disappear entirely, indicating the successful removal of most oxygen-containing groups in the GO. Moreover, in comparison with GNs, the FT-IR spectrum of PC−-GNs shows some new peaks at 1153 and 1205 cm−1 (C–H in-plane deformation vibration from aryl), and 1572 cm−1 (asymmetrically stretching vibration from COO−), implying the functionalization of GNs by the oxidation product of PCA. The weight percentage of PC− in the PC−-GNs is estimated to be about 20 wt% from the thermogravimetric analysis (TGA) result (figure S1 available at stacks.iop.org/Nano/24/395604/mmedia).
Figure 1. FT-IR spectra of GO (1), GNs (2) and PC−-GNs (3).
Download figure:
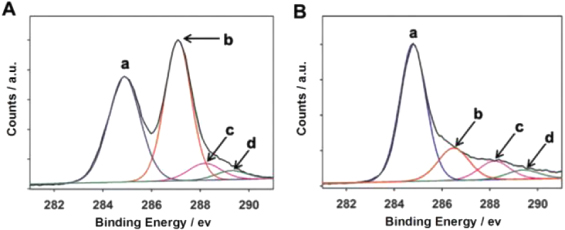
Standard image High-resolution imageA successful reduction of GO with PCA was also verified by XPS. Figure 2 shows the C 1s XPS spectra of GO before and after the reduction with PCA for 20 h. Before reduction (figure 2(A)), four peaks centered at 284.9, 287.1, 288.2, 289.3 eV, corresponding to C=C/C–C in aromatic rings, C–O (epoxy and alkoxy), C=O, and O–C=O groups [38], respectively, were detected in the GO. After reduction (figure 2(B)), the intensity of the peak of C–O (epoxy and alkoxy) (peak b), decreases dramatically, revealing that most of the C–O groups were removed. On the other hand, the relative contents of the various groups in GO and PC−-GNs, which are calculated from the areas of the contributing peaks in their fitting C 1s XPS spectra, are listed in table 1. It is noted that, in comparison with GO, the atomic percentage of the C=C/C–C group obviously increases and the atomic percentage of the C–O group decreases in the PC−-GNs, implying the recovery ofthe conjugated system and the removal of the C–O groups in the epoxy and alkoxy form after the reduction of GO. Moreover, the relative contents of both C=O and O–C=O groups in the PC−-GNs are higher than those in the GO. The increase of the contents of these groups comes only from the COO− group provided by the oxidation products of PCA molecules which adhere to the surface of reduced GNs by strong π–π stacking, further demonstrating carboxylate functionalization of GNs.
Figure 2. C 1s XPS spectra of GO (A) and PC−-GNs (B). The peaks of a, b, c and d correspond to C=C/C–C in aromatic rings, C–O (epoxy and alkoxy), C=O, and O–C=O groups, respectively.
Download figure:
Standard image High-resolution imageTable 1. Atomic percentage (at.%) of various groups in GO and PC−-GNs.
| C=C/C–C | C–O | C=O | O–C=O | |
|---|---|---|---|---|
| GO | 36.8 | 48.8 | 8.4 | 6.0 |
| PC−-GNs | 64.3 | 17.2 | 11.3 | 7.3 |
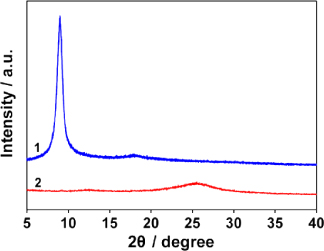
Figure 3 is the x-ray diffraction (XRD) patterns of GO and PC−-GNs. After the reduction, the sharp XRD peak of GO (d-spacing 9.8 Å at 2θ = 9.0°) disappears, but a new broad diffraction peak (d-spacing 3.5 Å at 2θ = 25.4°) appears, which is close to the typical (002) diffraction peak of graphite (d-spacing 3.4 Å at 2θ = 26.4°). The XRD results further confirm the reduction of GO by PCA.
Figure 3. XRD patterns of GO (1) and PC−-GNs (2).
Download figure:
Standard image High-resolution imageGenerally, the electronic conductivity of reduced GO can reflect both the extent of the reduction and the restoration of the electronic conjugation state. In order to measure the electronic conductivity of the PC−-GNs nanohybrid, a thin circular 'paper' (1 cm in diameter, 0.3 mm in thickness) of the PC−-GNs was prepared by filtration, and was dried under vacuum at 80 ° C for 12 h [38]. The electronic conductivity data was obtained by the four-point probe method. The average conductivity of PC−-GNs is about 1.8 S cm−1, which is comparable to the conductivity of the GO reduced by hydrazine and other reductants [20]; clearly indicating the successful reduction of GO via PCA.
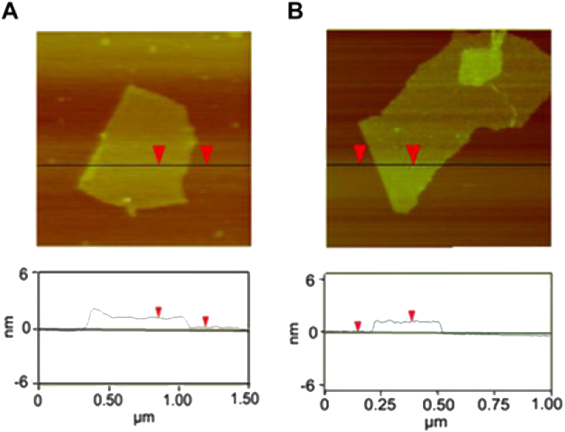
Figure 4 shows the typical AFM images of GO and PC−-GNs. The samples used for AFM studies were prepared by coating the corresponding dispersions on freshly cleaved mica surfaces. The AFM image of the GO (figure 4(A)) indicates that the mean thickness of GO sheets is about 1.07 nm, being close to the interlayer spacing of GO (0.98 nm) measured by XRD. On the other hand, the average thickness of the obtained PC−-GNs is about 1.24 nm (figure 4(B)). Taking into account that monolayered PC− molecules possibly cover both sides of the GNs by π–π stacking and the distance between PC− and the graphene sheet is ∼0.35 nm [39, 40], the average thickness of the GNs in PC−-GNs should be about 0.54 nm. This value is a little larger than the interlayer spacing of graphene (∼0.34 nm). Considering a low content of unreacted functional groups in PC−-GNs, and the overestimate of sheet thickness in AFM measurements, it is reasonable to conclude that these nanosheets are monolayers [41].
Figure 4. Tapping-mode AFM images and height analyses of GO (A) and PC−-GNs (B).
Download figure:
Standard image High-resolution imageIt is well known that the solubility of GNs in solvents is one of the key issues for its practical applications. As expected, the solubility of GNs in water is clearly improved when PCA is used as the reductant. It is noted that a black precipitate of GNs could be observed at the bottom of the bottle when GO was reduced with hydrazine (figure 5(A)). However, after reduction of GO with PCA, a stable and homogeneous black dispersion was obtained (figure 5(B)). The reasons should be as follows: the large amount of PC− resulting from the oxidation products of PCA introduce many carboxylate ions on the surface of the in situ reduced GNs by strong π–π stacking and hydrophobic interactions between the pyrene moiety and the basal plane of graphite. The surface-bound carboxylate ions not only greatly enhance the surface hydrophilicity of GNs, but can also prevent aggregation of GNs by electrostatic repulsion, resulting in a stable PC−-GNs dispersion in water.
Figure 5. Images of water dispersion (0.5 mg ml−1) of GNs (A) and PC−-GNs (B).
Download figure:
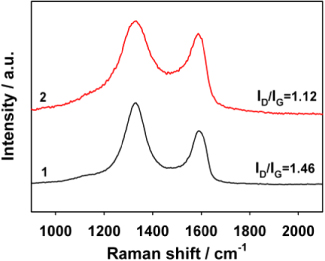
Standard image High-resolution imageRaman spectroscopy was employed to investigate the structural characteristics of GNs and PC−-GNs, and the corresponding results are shown in figure 6. The peak at ∼1330 cm−1 should be assigned to the A1g breathing mode of the disordered graphite structure (i.e., the D band) and the peak at ∼1590 cm−1 is ascribed to the E2g structural mode of graphite (i.e., the G band). The G band reflects the structure of the sp2 hybridized carbon atoms. The D band is due to defect sites in the hexagonal framework of the graphite materials [42]. The extent of the defects in graphite materials can be evaluated by the intensity ratio of the D to G bands (i.e., ID/IG). As shown in figure 6, the value of the ID/IG ratio is 1.12 for PC−-GNs and 1.46 for GNs. The obviously lower ID/IG ratio for PC−-GNs indicates that the aromatic conjugation structure of PC− bound to the basal plane of GNs is beneficial in shielding the defect sites in the reduced graphene nanosheets [43], which will be in favor of the applications of GNs, especially in electrocatalysis. Based on the good solubility, the plentiful uniformly distributed binding groups (carboxylate groups from the oxidation products of PCA) and the low number of structural defects, PC−-GNs should be a promising catalyst support for noble metal NPs in fuel cells.
Figure 6. Raman spectra of GNs (1), and PC−-GNs (2).
Download figure:
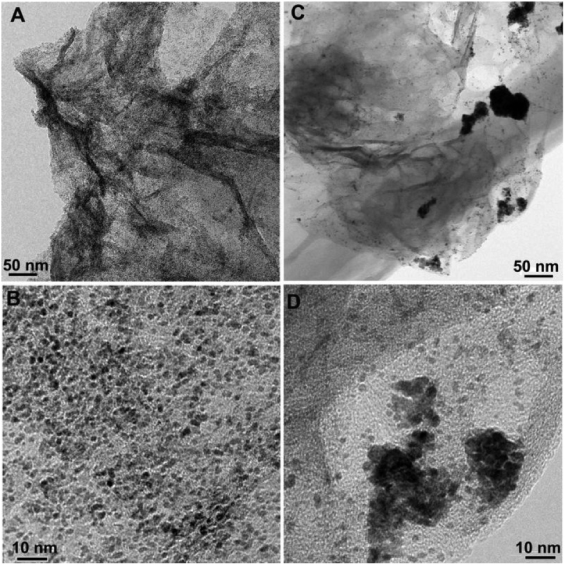
Standard image High-resolution imageFigure 7 shows the TEM images of Pt NPs/PC−-GNs and Pt NPs/GNs nanohybrids. As shown in figures 7(A) and (B), the surfaces of the PC−-GNs are successfully decorated with many fine and well-dispersed Pt NPs. Their size distribution was evaluated statistically by measuring the diameter of two hundred Pt NPs in the selected TEM image. The average diameter of Pt NPs is about 1.3 ± 0.2 nm, with a narrow distribution, mainly between 0.7 and 2.3 nm (figure S2(A) available at stacks.iop.org/Nano/24/395604/mmedia). It is noted that no obvious aggregation of NPs is observed on the surface of the PC−-GNs. However, for the Pt NPs/GNs nanohybrids, Pt NPs appear to be seriously aggregated on the surface of the GNs (figures 7(C) and (D)) and a broad particle size distribution (about 1.1–5.7 nm) with an average diameter of about 2.4 ± 0.5 nm (figure S2(B) available at stacks.iop.org/Nano/24/395604/mmedia) is observed. The reasons should be as follows: for the GNs, there were only a few nonuniformly distributed oxygen-containing functional groups remaining on the surface of the GNs after GO was reduced by hydrazine. When Pt NPs were deposited on the GN surface, the particles tended to deposit on the localized sites, thus resulting in poor dispersion and aggregation of the Pt NPs. However, for the PC−-GNs, many PC− were uniformly distributed on the surface of the GNs (scheme 1) via π–π stacking interactions. The carboxylate groups in PC− provided many binding sites to effectively anchor the precursor of the noble metal ions by coordination action [44]. Therefore, a much more uniform distribution of Pt NPs with small particle sizes was observed on the surface of the PC−-GNs.
Figure 7. TEM images of Pt NPs/PC−-GNs (A), (B) and Pt NPs/GNs (C), (D) nanohybrids.
Download figure:
Standard image High-resolution imageThe electrocatalytic performance of the Pt NPs/PC−-GNs and Pt NPs/GNs nanohybrids towards methanol oxidation was investigated. Cyclic voltammetry was used to study the electrocatalytic activity of the catalyst towards methanol oxidation in a nitrogen-saturated 0.5 M H2SO4 + 1.0 M CH3OH solution, and the corresponding results are shown in figure 8(A). In comparison with the Pt NPs/GNs catalyst, a significant enhancement of the peak current of methanol oxidation can be observed on the Pt NPs/PC−-GNs catalyst. It is noted that the forward peak current of methanol oxidation on the Pt NPs/PC−-GNs catalyst is 288.7 mA mg−1, almost 2.1 times higher than that on the Pt NPs/GNs catalyst (135.2 mA mg−1), due to the higher dispersion and smaller particle size of Pt NPs on the PC−-GNs. The electrocatalytic activity of the Pt NPs/PC−-GNs nanohybrids is also obviously improved as compared to the reported literature values [8, 45, 46], showing potential prospects in fuel cell applications. The long-term stability of these catalysts was also evaluated by chronoamperometry (CA), and the corresponding results are shown in figure 8(B). Within two hours of operation, although the current gradually decays for all catalysts, the Pt NPs/PC−-GNs catalyst shows a much slower deterioration rate than the Pt NPs/GNs catalyst, and has a much higher steady-state limiting current (5.4 mA mg−1) than Pt NPs/GNs (0.2 mA mg−1) after 2 h, indicating that the Pt NPs/PC−-GNs catalyst has a much better electrocatalytic stability than the Pt NPs/GNs [47]. This may be explained as follows: the hydrophilicity of the GNs surface can be increased by forming strong hydrogen bonds between water molecules and the carboxylate groups formed in the Pt NPs/PC−-GNs catalyst as the oxidation product of PCA. This will facilitate the dissociation of water molecules to produce –OHads during the methanol-oxidation reaction [48], thus resulting in the more efficient oxidation of the intermediate species usually generated in the methanol-oxidation reaction and a better resistance to the poisoning ability of the catalyst.
Figure 8. Cyclic voltammograms (A) at a scan rate of 50 mV s−1 and CA curves at 0.5 V (B) of Pt NPs/PC−-GNs (1) and Pt NPs/GNs (2) in nitrogen-saturated 0.5 M H2SO4 + 1.0 M CH3OH.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, a new reductant, PCA, was introduced to replace the typical reductant (hydrazine) in the chemical reduction of exfoliated GO. Based on the reducing ability of the aldehyde group in PCA, the strong π–π stacking action between PCA and GO, and the negatively charged and hydrophilic carboxylate group in the oxidation product (PC−) of PCA, a one-step reduction/functionalization strategy to produce highly reduced, water-soluble and carboxylate-functionalized GNs (PC−-GNs) has been developed. Compared with GNs obtained from the chemical reduction of GO by hydrazine, PC−-GNs provide a large number of binding sites (carboxylate groups) to anchor metal precursors and stabilize metal nanoparticles in the subsequent process, resulting in a high dispersion of Pt NPs with small particle sizes on the GN surface, superb electrocatalytic activity and good stability of the Pt NPs/PC−-GNs nanohybrids towards methanol oxidation.
Acknowledgments
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (20110161110009), Hunan Provincial Natural Science Foundation of China (12JJ2010), the Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation (No. 2013CL03), and Hunan Science and Technology Project (2009GK3015).