Abstract
Methods which disperse single-walled carbon nanotubes (SWNTs) in water as 'debundled', while maintaining their unique physical properties are highly useful. We present here a family of cationic cholesterol compounds (Chol+) {Cholest-5en-3β-oxyethyl pyridinium bromide (Chol-PB+), Cholest-5en-3β-oxyethyl N-methyl pyrrolidinium bromide (Chol-MPB+), Cholest-5en-3β-oxyethyl N-methyl morpholinium bromide (Chol-MMB+) and Cholest-5en-3β-oxyethyl diazabicyclo octanium bromide (Chol-DOB+)}. Each of these could be easily dispersed in water. The resulting cationic cholesterol (Chol+) suspensions solubilized single-walled carbon nanotubes (SWCNTs) by the non-specific physical adsorption of Chol+ to form stable, transparent, dark aqueous suspensions at room temperature. Electron microscopy reveals the existence of highly segregated CNTs in these samples. Zeta potential measurements showed an increase in potential of cationic cholesterol aggregates on addition of CNTs. The CNT–Chol+ suspensions were capable of forming stable complexes with genes (DNA) efficiently. The release of double-helical DNA from such CNT–Chol+ complexes could be induced upon the addition of anionic micellar solution of SDS. Furthermore, the CNT-based DNA complexes containing cationic cholesterol aggregates showed higher stability in fetal bovine serum media at physiological conditions. Confocal studies confirm that CNT–Chol+ formulations adhere to HeLa cell surfaces and get internalized more efficiently than the cationic cholesterol suspensions alone (devoid of any CNTs). These cationic cholesterol–CNT suspensions therefore appear to be a promising system for further use in biological applications.
Export citation and abstract BibTeX RIS
1. Introduction
Carbon nanotubes (CNTs) represent the true example of nanomaterials, because of their nanometer dimensions and unique electronic, thermal, optical and mechanical properties [1]. CNTs have also been investigated for their possible applications in diagnostic and therapeutic areas and in biotechnology [2, 3]. Their nanoscale dimensions render them to be excellent vehicles for the delivery of therapeutically active molecules like genes [4], toxins [5] and drugs inside the cells [6]. The natural ability of CNTs to absorb near-infrared (NIR) radiation and consequent heating of the backbone has been already utilized for achieving enhanced therapeutic effects by destroying selected disease causing cells via local heating effects [7].
After sorting out the cytotoxicity problems related to CNTs [8–11], they are under the scanner of scientists to be used as synergistic additives in gene delivery formulations [12]. The development of efficient non-viral gene delivery vectors is at the center of attention for the practical use of gene therapy [13]. Varieties of molecules have been examined in this context and molecular level structural variations in such designed molecules bring about improvements in the gene-transfer activity [14]. Furthermore, incorporation of natural transfection enhancers and other additives in pre-formed formulations [15–18] and incorporation of nanoparticles and other nanomaterials promote enhanced gene delivery across cells, resulting in a therapeutic effect. Moreover, the intrinsic properties of nanomaterials could be useful in additional applications besides gene delivery [19–21]. Accordingly nanomaterials are of intense interest for research investigations as they offer great promise for the biomedical applications [22–28]. There are several reports that describe the applications of CNTs and functionalized CNTs towards efficient drug [29, 30] as well as gene delivery [31]. Some of the major issues related to the CNT-based gene transfection recipes include the maintenance of solubility of carbon nanotubes in aqueous medium, stability of the resulting formulations and the retention/enhancement of photophysical and biophysical properties. CNT-assisted gene delivery brings about additional issues that come under close scrutiny such as binding with genes (DNA complexation), release of the genetic material from such complexes, their stability in blood serum, endosomal escape, size of the complex particles and their biocompatibility, etc. Even though CNTs possess strong potential for biomedical applications, their usefulness in this area is often limited by their lack of solubility in most of the solvents [32] and particularly in water, the obvious medium for biological studies.
Accordingly intense efforts are underway to render CNTs water-soluble using either surfactants [33], large biomolecules, e.g. DNA, proteins [34], etc, or suitably functionalized aromatic organic molecules [35, 36]. However, the surfactant (e.g. SDS, SDBS)-solubilized CNTs cannot be used in biological systems due to the highly toxic nature and the cell rupturing properties of such surfactants. Moreover, the high surfactant concentrations (millimolar range) required to solubilize the CNTs in the reported instances restrict their utility in truly biological systems even further. Virus-mediated solubilization of CNTs is not desirable because of the possible alteration in properties of the chosen biological systems [37] and also due to their adverse immunogenic response. Certain water-soluble pyrene-based compounds are highly effective in solubilizing CNTs. But these are of limited use due to their potent carcinogenic properties [38].
Thus, there is an urgent need to efficiently solubilize the CNTs in water using suitable molecules at a sufficiently low concentration at which the toxicity must be insignificant. Ciofani et al have reported the use of cholesterol as one of the components in cationic lipid mixtures to furnish CNT suspensions [39]. However, it was possible to suspend only low % (w/w) of CNT in a very complex mixture of lipidic molecules in this instance. In a separate effort Ciani et al considered the possibility of using CNTs as a device to trap and remove cholesterol from a living organism. Accordingly these authors performed ab initio calculations to determine how cholesterol interacts with CNTs. It was found that cholesterol as such exhibits no particular affinity towards a bare CNT. However, the CNT binding can be increased by including the cholesterol with a cation. Thus the presence of Ca2+ ions on the walls of a CNT increases the binding energy of cholesterol to a CNT by around 1.5 eV, regardless of the nanotube's diameter.
In view of the above reports, herein we attempt the dispersion of single-walled CNTs (SWCNTs) in water using certain cationic cholesterol derivatives for the first time. Compared to SDS or SDBS, very low concentrations of such cationic cholesterols are required for the solubilization of significant amounts of CNTs. We also include the characterization of the above CNT–lipid suspensions using Raman, circular dichroism spectroscopy, dynamic light scattering measurements, field emission scanning and transmission electron microscopy. These confirm that the integrity of CNTs remains intact upon the above optimized solubilization procedure. Compared to the cationic cholesterol suspensions alone, the CNT-loaded cationic cholesterol suspensions bind to duplex DNA more efficiently, leading to complex formation. The release of DNA from these CNT-loaded complexes was not affected at all, as in the case of the complexes that did not contain CNTs. Importantly the CNT-loaded complexes showed better stability in fetal bovine serum (FBS) media than the complexes that did not contain CNTs. Further, the confocal microscopy studies confirm that these CNT-loaded cationic cholesterol suspensions adhere to HeLa cell surfaces efficiently and get internalized better than their parent formulations devoid of CNTs.
2. Experimental details
2.1. Materials and methods
The single-walled carbon nanotube (SWCNT) used in this study was procured from HiPCO and was used as received. UV–vis–NIR spectra of SWCNT as aqueous suspension were recorded on a Perkin Elmer Lambda 35 spectrophotometer. For Raman spectra, samples were prepared by depositing a thin film on a glass slide and measured in a Horiba Jobin Yvon instrument. Zeta potential measurement was performed on a 90Plus Brookhaven particle sizer. Field emission scanning electron microscopy (SEM) images were taken on an FEI-Quanta 200 instrument. Transmission electron microscopy (TEM) images were taken on a JEOL 200-CX instrument. CD spectra were recorded on a JASCO J-815 CD Spectrometer Model J-815-150S. A Hitachi F-4500 fluorescence spectrophotometer was used for the fluorescence studies. A Trans-sonic T 460H Elma bath sonicator was used for sonication at a power setting of 35 kHz. A DynaPro molecular sizing instrument (model no. DYNAPRO-E-50-830) together with DYNAMICS V6(tm) ver. 6.3.40 software was used for the determination of the hydrodynamic diameter of various aggregates. CT-DNA was obtained from Cisco Research Laboratories, India.
2.2. Synthesis
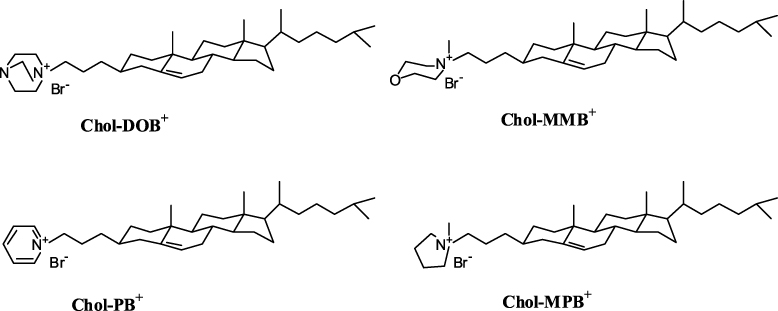
Cationic cholesterol compounds (Chol+) (figure 1) were synthesized following a procedure from the literature [40]. Briefly reaction of the cholest-5-en-3β-oxyethane bromide with the corresponding amine in dry MeOH: EtOAc (4 ml, v/v: 1/1) in a screw-top pressure tube gives >80% yield of all four cationic cholesterols. Each final compound was purified by repeated crystallization from a mixture of MeOH and EtOAc and characterized by 1H-NMR, HRMS and elemental analysis (supporting information available at stacks.iop.org/Nano/23/065101/mmedia).
Figure 1. Molecular structures of the cationic cholesterol compounds used in this study.
Download figure:
Standard image2.3. Preparation of cationic cholesterol suspensions in water
A given cationic cholesterol compound (Chol+) (∼0.5 mg) was dissolved in chloroform in autoclaved Wheaton glass vials. Thin films were made by evaporation of the organic solvent under a steady stream of dry nitrogen. Final traces of CHCl3 were removed by keeping the film under vacuum overnight. Freshly autoclaved water (Milli-Q) was added to each film such that the final concentration of the cationic cholesterol was 0.5 mM. Each mixture was kept for hydration at 4 °C for 10–12 h and was repeatedly freeze–thawed (ice-cold water to 60 °C) with intermittent vortexing to ensure optimal hydration. Dispersal of these suspensions for 15 min in a bath sonicator at 60 °C afforded Chol+ aggregates. These aggregates were stable and, if stored frozen, possessed a shelf life of several months.
2.4. CNT solubilization
CNTs were dispersed by sonication of a mixture of a solution of a given cationic cholesterol and HiPCO SWCNTs. A solution of each compound (1.66 mM) was prepared in water (blank solution). The procedure of CNT solubilization was similar for each cholesterol derivative. A representative description is given below. Briefly SWCNTs (400 μg) were added to an aqueous suspension of Chol–MMB+ (1 ml, 1.66 mM) and the mixture was bath-sonicated at room temperature for 15 min to form a suspension. This solution was kept at room temperature for 24 h and then bath-sonicated for 30 min again at identical settings. CNTs were solubilized to afford a black suspension from Chol–MMB+. Centrifugation of the suspension at 7.2 × 103g for 10 min afforded a clear supernatant which was collected by a pipette. The residue was again sonicated with a fresh Chol–MMB+ solution (300 μl, 1.66 mM) for 30 min and then centrifuged again at 7.2 × 103g for 10 min. Supernatant was again collected by a pipette. This step was repeated again to solubilize the remaining CNTs from the residue. Additional Chol–MMB+ solution (600 μl, 1.66 mM) was added to CNT–Chol–MMB+ suspension (400 μl) and sonicated further for 1 h. This suspension was then centrifuged at 9.8 × 103g for 20 min, which left practically no residue, giving a clear transparent CNT–Chol–MMB+ suspension (final CNT conc. 9% w/w in aqueous solution containing 1.66 mM Chol–MMB+) (figure 2). This CNT-loaded suspension (stock solution) was used for all further studies. With other cationic cholesterol compounds, a similar procedure was used for complete CNT solubilization.
Figure 2. Single-walled carbon nanotubes (SWCNT) dispersed in water by various cationic cholesterol compounds (Chol+) (∼0.4 mg ml−1) at room temperature. CNTs were solubilized to a lesser extent (∼0.2 mg ml−1) in Chol–MPB+ than other Chol+ suspensions.
Download figure:
Standard image2.5. UV–vis–NIR spectroscopy
Each stock solution was diluted 20 times with deionized water to make the concentration of cationic cholesterol in the suspension at ∼83 μM. UV–vis–NIR spectroscopy of this sample was recorded from 1100 to 200 nm wavelength range by taking the suspension in quartz cuvettes. Spectra were recorded for the individual suspensions of each cholesterol compound with CNTs and compared against the samples without CNTs (i.e. blank cationic cholesterol suspension).
2.6. Circular dichroism (CD)
CD spectra of the suspension of each cholesterol compound were recorded either at 83 μM or its half-concentration at 41.5 μM in the wavelength range of 500–190 nm against pure water as reference (supporting information, figure S1 available at stacks.iop.org/Nano/23/065101/mmedia). CD for the CNT–Chol+ suspension was recorded under similar conditions as for pure cationic cholesterol. For recording every CD spectrum of a CNT–Chol+ suspension, the concentration of the cationic cholesterol was kept constant at 83 μM while the CNT concentration was increased gradually from the stock solution in subsequent measurements.
2.7. Dynamic light scattering studies
CNT–Chol+ suspensions (1.66 mM in terms of Chol+) were prepared in pure water (Millipore) as mentioned in the CNT solubilization procedure. The suspensions were diluted to 0.33 mM (in terms of Chol+) and were subjected to dynamic light scattering (DLS) measurements, using a molecular sizing instrument (model no. DYNAPRO-E-50-830), which employed an incident laser beam of ∼830 nm wavelength. The values reported are the averages of four independent experiments, each of them having 10 sub-runs.
2.8. Zeta potential measurements
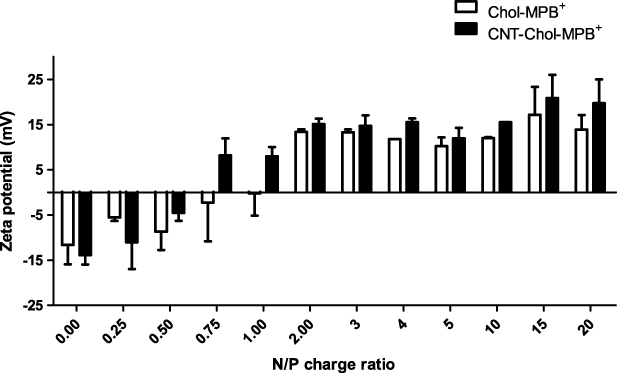
Zeta potential of an aqueous suspension of Chol–MPB+ and the corresponding CNT-loaded suspension, CNT–Chol–MPB+, was measured on a 90Plus Brookhaven particle sizer. The stock concentration of Chol–MPB+ was 1.66 mM in all the samples while keeping CNT constant (9 wt%). The experiment was performed using calf-thymus DNA (CT-DNA) (28.7 μM, base molarity). Initially the zeta potential was measured for the DNA solution (2 ml) in deionized water. Then the changes in zeta potentials were followed by each addition of Chol–MPB+ or CNT–Chol–MPB+ suspensions, respectively.
2.9. Transmission electron microscopy (TEM)
For the preparation of CNT–Chol+–DNA composites, 4 μl of CT-DNA (1 mM base pair molarity) was added to a 5 μl of CNT suspension in water containing 1.66 mM cationic cholesterol such that the Chol+–DNA ratio reached a value of 2:1. This was then incubated for 30 min. A similar procedure was followed for each CNT–Chol+ and CNT–Chol+–DNA suspensions for preparation of the sample for TEM studies. A 10 μl sample of the suspension was loaded onto a Formvar-coated 400-mesh copper grid and allowed to remain for 1 min. Excess fluid was wicked off the grids by touching their edges to a filter paper. Then 10 μl of 0.1% uranyl acetate was applied on the same grid after which the excess stain was wicked off and the grid was air-dried for 30 min. Each sample was then observed under TEM (JEOL 200-CX) operating at an acceleration voltage (DC voltage) of 100 keV. Micrographs were recorded at a magnification of 4000–20 000 × . Similarly freshly prepared aqueous suspensions of each Chol+, CNT–Chol+ and CNT–Chol+–DNA complexes were examined by negative staining as described above.
2.10. Scanning electron microscopy (SEM)
Each CNT–Chol+–DNA suspension was prepared as described above. An aliquot of this suspension (20 μl) was scooped on a brass stub and the sample was kept for 5 h at room temperature to air dry. This was followed by freeze drying for another 5 h. Similarly, CNT–Chol+ samples were prepared without DNA and were used for the SEM study on a Quanta 200 SEM operated at 5 kV.
2.11. Ethidium bromide displacement assay
In this study intercalator ethidium bromide (EB) displacement from duplex DNA was utilized as a measurement of each cationic cholesterol's ability to condense duplex DNA in the absence or presence of CNTs. Fluorescence emission at 592 nm due to EB (2.5 μM) in 5 mM HEPES, 40 mM NaCl, pH 7.4 buffer was monitored in a Hitachi model F-4500 spectrofluorimeter (λex = 526 nm). To this CT-DNA (4 μM) was added and the change in emission due to EB upon intercalation inside duplex DNA was measured at 37 °C. Then aliquots of a given cationic cholesterol or CNT–Chol+ suspension (1.66 mM) were incrementally added into pre-formed EB/CT-DNA intercalated complexes. This resulted in fluorescence quenching due to the displacement of EB molecules from the intercalation sites of duplex DNA and finally reached saturation. This was caused due to the distortion in duplex DNA organization brought about upon addition of cationic cholesterol or CNT–Chol+ aggregates.
To probe the stability of such Chol+–DNA or CNT–Chol+–DNA complexes, anionic SDS solution (0.9 mM) was added incrementally to Chol+–DNA or CNT–Chol+–DNA complexes formed. This resulted in the partial recovery of the fluorescence emission at 592 nm due to the release of double-helical DNA from either Chol+–DNA or CNT–Chol+–DNA complexes. The released DNA retains its native conformation and re-intercalates EB, resulting in an increase in the fluorescence emission. If F0 is the fluorescence intensity (FI) of non-intercalated and Fmax is the FI of fully intercalated EB, and Fx is the FI for a given concentration of Chol+ compound or SDS, then % FI is expressed as

In the same way we also examined the stability of Chol+–DNA and CNT–Chol+–DNA complexes in the presence of serum. We performed this experiment essentially in a similar manner as described for EB intercalation assay except that, rather than using anionic SDS solution to induce DNA release and EB re-intercalation, we added here FBS (fetal bovine serum) solution progressively. Addition of serum to Chol+–DNA or CNT–Chol+–DNA complexes resulted in the destabilization of the latter and release of DNA from the Chol+–DNA and CNT–Chol+–DNA complexes. Due to the release of duplex DNA, EB re-intercalated and fluorescence emission increased. Based on equation (1), we estimated the stability of CNT–Chol+–DNA complexes in the presence of various amounts of serum.
2.12. Cell adherence and internalization of the CNT–Chol+ formulation
To examine the cell binding and internalization efficiency of different cationic cholesterol suspensions and their CNT-loaded formulations, we performed confocal microscopy using HeLa cells. In brief, cells were cultured in T25 culture flasks, trypsinized and plated in 12-well plates, having autoclaved glass slips in wells. Nearly, 60 000 cells per glass cover slip were plated in antibiotic-free 10% FBS containing DMEM medium as cells remained on glass slips. Cells were grown for 24 h at 99% humidity at 37 °C and 5% CO2 condition till the cell monolayer gained ∼70% confluence. Experiments performed using Chol–DOB+ and CNT–Chol–DOB+ are described in detail below.
Working stocks of the Chol–DOB+ formulation were prepared in plain DMEM. Separately diluted fluorescein isothiocyanate (FITC) conjugated rabbit antibody (anti-rabbit-FITC) and desired amounts of Chol–DOB+ and CNT–Chol–DOB+ suspensions were mixed in a total volume of 200 μl of DMEM alone and incubated at room temperature for about 30 min to prepare anti-rabbit-FITC-Chol–DOB+ and anti-rabbit-FITC–CNT–Chol–DOB+ complexes. After 30 min of complexation, 200 μl of plain DMEM was added to the complex and the old medium was removed from the wells followed by washing of the cells with DMEM. Anti-rabbit-FITC–Chol–DOB+ and anti-rabbit-FITC–CNT–Chol–DOB+ complexes in 200 μl media per well were added to the cells. Then plates were incubated for 3 h at 37 °C in a 99% humidified atmosphere containing 5% CO2. At the end of the incubation period, the medium was removed and cells were washed with DPBS buffer properly and all the cell debris was removed carefully without disturbing the monolayer of cells. Then cells were fixed for 10 min with paraformaldehyde (4% in DPBS) using 1 ml/well. Cells were washed with 1 ml DPBS for 3 × 10 min. Then cells were kept with 1 ml Triton-X-100 (0.1%) for 5 min to increase the membrane permeabilization. Again cells were washed with DPBS for 3 × 10 min. Then glass slips were fixed and permealizable monolayers of HeLa cells were taken out from the wells and kept on the glass slides and incubated for 5 min with 1 μg ml−1 of PI (propidium iodide) to specifically stain the nucleus of the cells. Again cells were washed with 1 ml DPBS for 3 × 10 min to remove the extra PI and to reduce over-staining. A vector shield was used to mount the cell-possessing glass slips on glass slides. Finally all the samples were examined under a confocal microscope (Zeiss LSM 510-Meta Apochromat).
3. Results
The aqueous suspensions (figure 2) of each of the cationic cholesterol compounds were prepared by repeated freeze–thawing (ice-cold water to 60 °C) of the hydrated compounds with intermittent vortexing. Then the resulting mixture was dispersed by bath sonication (35 kHz) at 70 °C for 15 min. Each cholesterol compound could be dispersed in water easily and a stable optically transparent solution in water was obtained. No precipitation or any increase in turbidity was observed even after several months when these solutions were stored in closed vials at 4 °C under sterile conditions. Chol–MPB+ was less soluble compared to other cationic chol+s and required sonication for >20 min for effective solubilization that was stable. The ease of solubilization and solubility order of cationic chol+s was observed to be Chol–MMB+ > Chol–DOB+ > Chol–PB+ > Chol–MPB+.
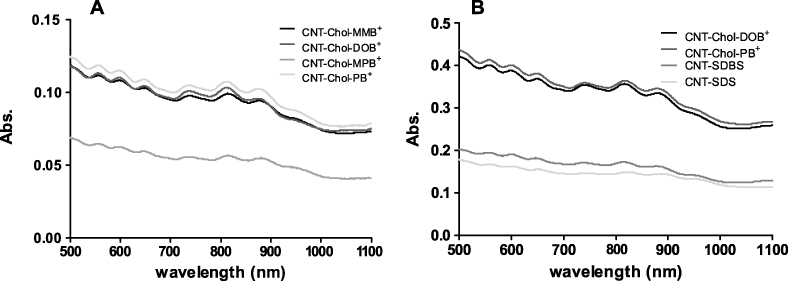
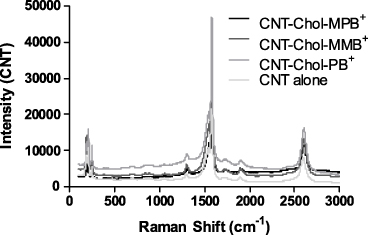
Aqueous suspensions of each cationic cholesterol (conc. ∼1.66 mM) were used for the solubilization of SWCNTs [41]. The dispersal of CNTs required their prior hydration in cationic cholesterol suspension. Each suspension containing CNTs showed characteristic vis–NIR spectral pattern due to the presence of SWCNTs in aqueous medium. Individual CNT–Chol+ showed variations in absorbance values depending upon the amount of CNTs solubilized in them but their 'fine-structured' signature in the region of 1100–500 nm was always retained in the vis–NIR spectrum. The extent of CNT loading in Chol+ suspensions was low in the case of Chol–MPB+ compared to other cationic cholesterol compounds as indicated from the vis–NIR spectrum (figure 3(A)). The observed broadening of the SWNT absorption spectra could be due to sonication of the samples for prolonged periods as recorded earlier [42]. Raman spectra showed similar patterns with pristine CNTs and that of its suspensions in various cationic chol+s (figure 4), suggesting that there was no breakage in the CNT backbone upon solubilization.
Figure 3. Vis–NIR spectra of (A) SWCNT–Chol+ suspensions. An aliquot of 50 μl of final mixture of each CNT–Chol+ compound (1.66 mM) was diluted using water up to 1 ml. CNT was solubilized in approximately equal amounts by each Chol+ suspension except for Chol–MPB+. (B) Solubilization efficiency of CNT by Chol+ or SDS or SDBS solutions in water. Concentrations of Chol–DOB+, Chol–PB+, SDS and SDBS used for solubilization of SWCNT (9% w/w) were 1 mg ml−1, 1 mg ml−1, 20 mg ml−1 and 5 mg ml−1, respectively. Vis–NIR spectra for the CNT–Chol+ suspension were recorded using 1/10 dilution.
Download figure:
Standard imageFigure 4. Raman Spectra of CNT and CNT–Chol+ suspensions. Neat CNT and its dispersions in Chol+ suspensions show similar Raman spectral peaks (G band ∼ 1580 cm−1) and at 2600 cm−1 (overtone of D band).
Download figure:
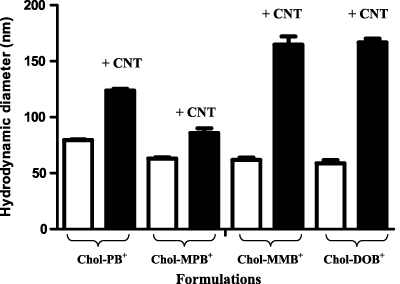
Standard imageA comparison of hydrodynamic diameters (DLS) of cationic cholesterol aggregates (0.33 mM) in water and their CNT-loaded aggregates is shown in figure 5. CNTs in the presence of cationic cholesterol generate flocculates with large hydrodynamic radius and high volume fraction as reported earlier [43]. Clearly the inclusion of CNTs into cationic cholesterol suspensions led to significant increases in their hydrodynamic radii as revealed from the DLS studies.
Figure 5. Comparison of hydrodynamic diameters of CNT–Chol+ suspensions. Bars represent the average sizes obtained from four independent experiments. In each Chol+ suspension, the Chol+ concentration was 0.33 mM and 9% (w/w) CNT was loaded into it.
Download figure:
Standard image3.1. Circular dichroism spectroscopy
Cholesterol-based cationic compounds, Chol–PB+, Chol–MPB+, Chol–MMB+ and Chol–DOB+, are chiral and showed characteristic signatures at 190–230 nm in each of their CD spectra (supporting information, figure S1 available at stacks.iop.org/Nano/23/065101/mmedia). However, none of these solutions showed any significant CD bands in the 240–290 nm range upon variation in the Chol+ concentration. On CNT solubilization, a specific band appeared in the 240–290 nm range, which was only found to be CNT concentration-dependent and this increased with increase in the CNT concentration. It showed a 'double hump'-like spectral signature in this region suggesting a preferential solubilization of chiral forms of CNTs in the above suspensions (figure 6).
Figure 6. CD spectra of Chol+ suspensions (83 μM) in which the indicated amount of SWCNTs was solubilized.
Download figure:
Standard image3.2. Characterization of neat CNT–Chol+ suspensions
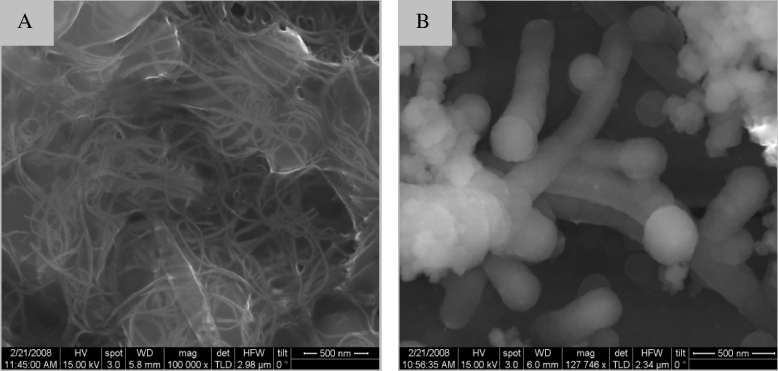
All CNT–Chol+ suspensions in water were found to be very stable. The CNT suspensions formed from each cationic cholesterol compound were transparent and black in color. CNT–Chol+ suspensions were characterized by DLS, zeta potential measurement, SEM and TEM. The sizes of the CNT–Chol+ aggregates were obtained from the DLS studies (figure 5, supporting information, table S1 available at stacks.iop.org/Nano/23/065101/mmedia). CNT–Chol–MMB+ suspensions showed largest aggregates in size whereas the aggregates in CNT–Chol–MPB+ suspensions were smallest in size. The zeta potential measurements of the resulting suspension (figure 7) clearly indicate that inclusion of CNTs enhances the electrophoretic potential of the aggregates [44, 45]. Thus the zeta potential of Chol–MPB+ was enhanced on inclusion of CNTs. Chol–MPB+ showed the isoneutrality point between an N/P ratio of 1–2 whereas in the case of CNT–Chol–MPB+ this point was between 0.5 and 0.75. Probably, in the aggregate state, Chol–MPB+ did not expose all the charges on the surface but, in the presence of CNT, those charges get exposed on the surface and show higher electrophoretic potential. SEM images showed the increase in segregation of CNTs upon stabilization in cationic chol+ suspension (figure 8).
Figure 7. Changes in the electrophoretic potential of a CT-DNA solution (conc. 28.7 μM base molarity) observed upon incremental addition of Chol–MPB+ alone or CNT-loaded Chol–MPB+ suspensions.
Download figure:
Standard imageFigure 8. FESEM images of (A) SWCNT alone and (B) CNT–Chol–PB+ suspension in water at 200 000 × magnification.
Download figure:
Standard image3.3. Chol+–DNA complexes
CNT–Chol+–DNA complexes were characterized both by SEM and TEM. Representative micrographs are shown in figures 9 and 10. These show distinguishable morphologies for CNT–Chol+–DNA complexes compared to those of CNT–Chol+ aggregates. The TEM images show the presence of 'inter-connected' aggregate networks (figure 10(a)). Higher magnification reveals that this network is simply an array of nanometer-sized entities arranged in a 'dendritic' fashion. A closer look at higher magnification also clarified the rod-like bundles of CNTs present in the dendritic network of CNT–Chol+–DNA complexes (figure 10(b)).
Figure 9. SEM images of representative CNT–Chol+–DNA complexes. (A) CNT–Chol–MMB+–DNA at 100 000 × and (B) CNT–Chol–PB+–DNA at 120 000 × magnification.
Download figure:
Standard imageFigure 10. TEM images of representative CNT–Chol+–DNA complexes with negative stain of uranyl acetate (0.01%) (a) CNT–Chol–MMB+–DNA and (b) CNT–Chol–PB+–DNA.
Download figure:
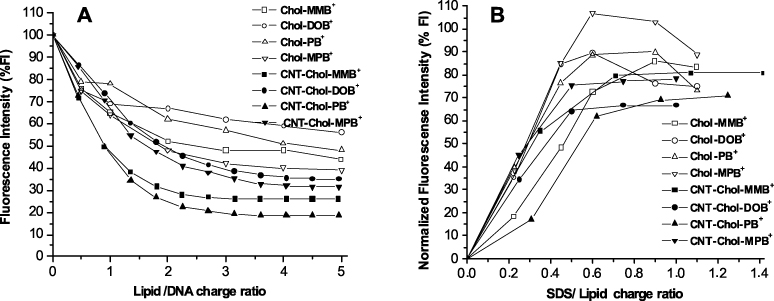
Standard imageWe probed the efficiency of DNA complexation by the cationic cholesterols in water in the absence and presence of CNTs using fluorescence experiments by exposing a duplex DNA solution to ethidium bromide (EB), where EB acts as a fluorescent probe via intercalation with the DNA base pairs. To a fully intercalated DNA sample, incremental addition of either Chol+ or CNT–Chol+ suspensions led to gradual quenching of the EB fluorescence. It reached a saturation value beyond a concentration of a given Chol+ or the corresponding CNT–Chol+ suspension. This quenching of the fluorescence intensity (FI) at 592 nm indicates the successive release of EB from the EB/DNA complex, as DNA binds strongly with cationic Chol+ or CNT–Chol+ species, leading to a compaction of DNA structure. In such a situation, DNA loses its intrinsic property of intercalation of EB. The percentage decreases in FI (FI%) till saturation for a given Chol+ aggregate were also dependent on the molecular structure of the Chol+ used. Thus both the molecular structure and the morphology of Chol+ suspensions effectively determined the % decrease in the fluorescence intensity on successive addition of Chol+ and Chol+–CNT formulations (figure 11).
Figure 11. (A) Ethidium bromide (EB) exclusion assay for neat Chol+ and CNT-loaded Chol+ suspensions. The release of the EB from the DNA–EB complexes induced upon addition of the neat Chol+ (empty symbols) and CNT–Chol+suspensions (filled symbols) at different Chol+/DNA charge ratios. (B) Comparative SDS-induced EB re-intercalation assay for neat Chol+ (empty symbols) and CNT–Chol+ suspensions (filled symbols). EB re-intercalation to the 'released' DNA from Chol+–DNA and CNT–Chol+–DNA 'cholaplexes' after the addition of anionic SDS at various SDS/Chol+ charge ratios is shown.
Download figure:
Standard imageNotably, each cationic cholesterol suspension loaded with SWCNTs showed higher efficiency in EB exclusion compared to the corresponding cationic cholesterol suspension only. However, even in the presence of CNTs, the molecular structure of the cationic cholesterol mattered in the efficiency of DNA complexation. Among all the CNT-based formulations, CNT–Chol–DOB+ showed minimum efficiency in ejecting the intercalated EB from DNA, resulting in a loss of ∼60% emission. CNT–Chol–PB+ was found to displace the maximum amount of EB from the DNA complexes, displacing nearly 80% at the N/P ratio of 2–3. The N/P values for each cationic cholesterol suspension were plotted as the % decreases in FI and are shown in figure 11(A). The % decreases in FI measured under the same conditions is a direct measure of the strength of the cationic cholesterol–DNA complex [39]. Interestingly, among the Chol+-only formulations, Chol–PB+ was the third best in excluding EB from DNA. However, it was rendered the most efficient when CNT was included in its suspension in water. Chol–MPB+, which was the best towards DNA complexation without CNT, became the second least efficient when complexed with DNA in the presence of CNTs. Suspensions of Chol–MMB+ and Chol–DOB+ remained comparable in this respect. Another point to be noted here is that all cationic cholesterol suspensions after solubilizing CNTs, enhanced their EB exclusion efficiency to different extents. Thus CNT–Chol–DOB+ excluded ∼70% from ∼45%, CNT–Chol–MMB+ changed to ∼70% from 55%, CNT–Chol–PB+ changed to ∼80% from 50% and CNT–Chol–MPB+ changed to ∼70% from ∼60%. This suggests that each chol+ interacts uniquely with CNTs depending on their molecular structure and resulting CNT–Chol+ aggregates manifest certain differences in their organization and surface properties upon aggregation. In other words, some cationic cholesterols wrap around the CNTs nearly completely while other derivatives leave some CNT surfaces unwrapped. Thus there was only ∼10% increase in the EB exclusion efficiency on addition of CNTs in Chol–MPB+ while other cationic cholesterols showed ∼15–25% increase. Probably it is due to less efficient CNT solubilization by Chol–MPB+ compared to other cationic cholesterol suspensions. This suggests that DNA not only binds to Chol+ wrapped on CNTs but 'exposed' regions of CNTs themselves too possess significant affinity for DNA. DNA may adhere to the exposed regions of CNTs due to the presence of its aromatic bases via π–π stacking interactions and indeed DNA binding to CNTs has been reported already [37].
3.4. SDS-induced release of DNA from cationic cholesterol–DNA complexes
After investigating the DNA binding by cationic chol+ aggregates, the other important issue is to understand whether such chol+ formulations are also able to release DNA in its native form from their complexes [46]. This is a prerequisite for its successful gene delivery into the cell. It is known that, in cell membranes, several negatively charged lipids are present in the endosomal layers, which trigger the DNA release from the cationic lipid–DNA lipoplexes by competing with DNA for their binding with cationic lipids [47, 48]. They possess stronger affinity to cationic lipids compared to DNA and replace DNA from such lipoplexes [49]. Probably it is due to the operation of both electrostatic and hydrophobic interactions in the case of cationic and anionic lipids while lipid and DNA are held together in their lipoplexes primarily through electrostatic interactions [50].
By taking the above facts into consideration, we determined the extent of DNA release induced by a model anionic micellar aggregates from its complexes with individual Chol+. Here we have used negatively charged SDS micelles to induce the release of DNA from various Chol+–DNA as well as CNT–Chol+–DNA complexes. As discussed earlier, excitation of an aqueous solution of EB at 526 nm gives a broad emission λmax at 592 nm. On addition of saturating concentration of duplex DNA to it the FI value was increased by ∼5-fold. Addition of Chol+ or CNT–Chol+ formulations to the EB-bound DNA caused release of EB from the complex. It quenched the FI depending on the concentration of Chol+ in formulations. On its progressive addition a saturation minimum of fluorescence intensity was reached. Addition of anionic SDS to this mixture induced dissociation of DNA from their DNA bound complexes of Chol+ or CNT–Chol+. This allowed the re-intercalation of the 'released' EB into the 'free' DNA which resulted in the increase in the FI on successive addition of SDS, eventually reaching a limiting value. Higher extent of increase in FI indicated greater re-intercalation of EB inside duplex DNA, which in turn suggests a release of more DNA from Chol+–DNA and CNT–Chol+–DNA complexes (figure 11(B)).
Examination of the profiles obtained by SDS-mediated DNA release from each Chol+–DNA and CNT–Chol+–DNA complex reveals significant differences in their ability to induce DNA release from such complexes, depending on whether CNT was present in them or not. Firstly it shows that each Chol+–DNA or CNT–Chol+–DNA complex requires different amounts of SDS to enable maximum release of DNA. Even the extent of maximum release depends on the molecular structure of the Chol+ in the Chol+–DNA and CNT–Chol+–DNA complexes. Interestingly, each formulation that included CNT induced less DNA release, as evident from lower FI recovery, compared to the corresponding Chol+ suspension without CNT, i.e. the Chol+–DNA 'cholaplex'. Chol+–DNA complexes showed ∼80–100% recovery of FI while CNT–Chol+–DNA complexes showed only ∼60–80% FI recovery depending on the molecular structure of Chol+ employed. It also corroborates the DNA binding with CNTs in CNT–Chol+–DNA complexes in addition to its DNA binding to the cationic cholesterol itself. Chol–MPB+–DNA complex showed the maximum (∼100%) FI recovery on addition of SDS while DNA-bound CNT–Chol–DOB+ complex showed only ∼60% FI recovery, which was the minimum in the series. Although DNA binding efficiency was maximum (∼80%) for CNT–Chol–PB+, its DNA release was only ∼70% while Chol–PB+ which showed the least (∼45%) DNA binding, its DNA release was as high as ∼90%. Similarly, CNT–Chol–MMB+ and CNT–Chol–MPB+ showed only ∼75% recovery, while the fluorescence from Chol–MMB+ recovered to the extent of ∼80% and ∼100% from that Chol–MPB+ although the extents of DNA binding to CNT–Chol–MMB+ and CNT–Chol–MPB+ were ∼70% and ∼60%, respectively. Hence, Chol+ formulations that contained CNT possessed better DNA binding ability compared to the formulations devoid of CNT.
3.5. Stability of Chol+–DNA and CNT–Chol+–DNA complexes in serum
After examining the DNA binding and release efficiency of the Chol+ suspensions with or without CNTs, we addressed another important issue pertaining to the stability of the Chol+–DNA and CNT–Chol+–DNA complexes in serum. To get an idea about the stability of the above complexes in FBS (fetal bovine serum) we again used the EB intercalation assay. As stated earlier, to the fully intercalated duplex DNA, addition of Chol+ or Chol+–CNT suspensions caused a release of EB from DNA leading to a fluorescence quenching according to the ability of individual Chol+ to effect DNA complexation. On progressive addition it reached a saturation of fluorescence lowering. In such a situation, progressive addition of FBS led to gradual increases in the fluorescence emission. It increased with increase in FBS concentration finally reaching a plateau. Increase in FI indicates re-intercalation of EB in DNA, which in turn gives a measure of the dissociation of DNA from the Chol+–DNA and CNT–Chol+–DNA complexes induced by the addition of FBS (figure 12).
Figure 12. Comparison of fetal bovine serum (FBS) induced release of DNA from its complexes with Chol+ versus CNT–Chol+ suspensions as a function of different % of FBS.
Download figure:
Standard imageExamination of the results obtained from FBS promoted DNA release from various Chol+–DNA and CNT–Chol+–DNA complexes also reveal significant differences. Notably all CNT–Chol+–DNA complexes showed better FBS stability compared to the Chol+–DNA complexes that did not contain any CNT. Thus the incorporation of CNTs in Chol+ suspension increases the stability of Chol+–DNA complexes in serum. Among all formulations, the CNT–Chol–MPB+–DNA complex possessed the maximum stability in FBS while the Chol–DOB+–DNA showed the minimum stability in FBS. Although CNT–Chol–MPB+–DNA complex has ∼70% stability in 10% of FBS, it showed only ∼70% DNA binding and ∼75% DNA release. Compared to this, CNT–Chol–PB+–DNA showed only ∼55% stability in FBS, but showed ∼80% DNA binding as well as ∼70% DNA release, while CNT–Chol–DOB+–DNA showed ∼75% stability in FBS, ∼70% DNA binding but only ∼60% DNA release. CNT–Chol–MMB+–DNA showed similar behavior as that of CNT–Chol–MPB+–DNA complex, i.e. ∼70% stability in FBS, 70% DNA binding and 75% DNA release, respectively. Thus each suspension without CNTs showed a consistently higher extent of DNA release compared to the respective suspension that contained CNTs. However, the former showed poorer DNA binding as well as FBS stability than the latter. Therefore CNT-containing Chol+-aggregates of DNA show better stability and tolerance to serum.
3.6. Enhanced binding and internalization of CNT–Chol–DOB+ in HeLa cells
Experiment was performed using anti-rabbit-FITC–Chol–DOB+ and anti-rabbit-FITC–CNT–Chol–DOB+ complexes. Complexes were clearly seen in confocal microscopy images where anti-rabbit-FITC–Chol–DOB+ were spread out to maximum in intracellular space (figure 13(A)) while anti-rabbit-FITC–CNT–Chol–DOB+ were either bound on the cell surface or internalized and located in cytoplasm (figure 13(B)). Figure 13(A) has been split into four panels, showing very little anti-rabbit-FITC–Chol–DOB+ bound to cell surface (panel (A3)) whereas a considerably greater extent of anti-rabbit-FITC–Chol–DOB+ is seen to adhere to cell surfaces (panel (B3)).
Figure 13. Confocal images showing enhanced binding and internalization of Chol–DOB+ in HeLa cells, when loaded with SWCNT (CNT–Chol–DOB+). Panel (A): anti-rabbit-FITC added to Chol+ and incubated for 30 min. Most of the formulations remain outside the cells and very little bind to the cell surface or internalize inside the cells; (A1) cells with PI staining; (A2) cells viewed under bright-field; (A3) anti-rabbit-FITC added to Chol–DOB+ suspension; (A4) overlaid panels A1 and A3 show little anti-rabbit-FITC binding to the cell surface; panel (B): anti-rabbit-FITC is added to CNT–Chol+ suspension and incubated for 30 min. Most of the formulations appear to bind to the cell surface as well as internalize inside the cells and very few remain outside the cells; (B1) cells with PI staining; (B2) cells under bright-field; (B3) anti-rabbit-FITC bound to Chol+ suspensions; (B4) overlaid panel (B1) and (B3) show maximum anti-rabbit-FITC binding with the cell surfaces.
Download figure:
Standard image4. Discussion
The cholesterol backbone in each compound is linked to different heterocyclic cationic polar groups via an ether type linkage (figure 1). For the present study, four cationic cholesterol compounds have been selected owing to their high propensity to form stable aqueous suspensions. The ease of solubilization and solubility order of these cationic cholesterols in water followed the order Chol–MMB+ > Chol–DOB+ > Chol–PB+ > Chol–MPB+. This solubilization order could be possibly due to the hydrogen bond forming capability and the polarity order of the head groups present in the above cholesterols. Chol–MMB+ and Chol–DOB+ have free O and N atoms, respectively, in the polar head group and can thus form hydrogen bonds with water molecules to enable the formation of a more stable suspension in water than Chol–PB+ and Chol–MPB+. Chol–PB+ is more soluble than Chol–MPB+, probably due to the induced polarity in the aromatic ring by cationic charge on the N atom of pyridine.
Single-walled CNTs could be easily dispersed in each of the above cationic cholesterol suspension [51] to produce stable, black, transparent suspension (figure 2). The CNT suspensions obtained by sonication followed by centrifugation were highly stable at room temperature, consisting of very pure SWCNTs as is evident from the UV–vis–NIR absorption spectroscopy (figure 3). The UV–vis absorbance spectral bands exhibited by SWCNTs in the NIR region originate from the electronic transitions between the first or second van Hove singularities of the CNTs [52–54]. The absorbance values of CNT present in different chol+ suspensions showed that these could solubilize ∼100 times more amount of CNT at the concentration of 0.1 mg ml−1 of Chol–PB+. Thus these could solubilize significantly higher amounts of CNTs compared to the amount of CNTs solubilized by SDA at 20 mg ml−1. The Raman spectrum of the pristine and solubilized CNTs have identical features for the G band at 1558 cm−1 (figure 4), indicating that the native skeleton of carbon nanotubes was intact in the aqueous suspension. It was also found that these CNT-loaded suspensions have higher zeta potential values compared to the cationic cholesterol suspensions alone, probably due to the reorganization of the aggregate structure upon CNT inclusion which exposed the buried positively charged head groups to the aqueous interface (figure 7). Also the suspensions induced 'debundling' of the CNTs. One can easily figure out the existence of the debundled CNTs in FESEM images of CNT–Chol–lipid suspensions (figure 8).
Based on the dynamic light scattering studies, it is clear that the aggregates formed in CNT-loaded Chol+ suspensions are larger in size than those of the respective Chol+ aggregates alone (supporting information, table S1 available at stacks.iop.org/Nano/23/065101/mmedia). This again suggests that the Chol+ molecules probably wrap around the CNTs and render the resulting complexes water-soluble. The CNT–Chol–DOB+ aggregates have the largest in diameter and the CNT–Chol–MPB+ aggregates have the smallest size. It is clear that Chol–MPB+s solubilize the minimum amount of CNTs and it correlates well with its hydrodynamic diameter data. Probably, both its size and solubility are affected to the minimum extent upon CNT incorporation suggesting the low affinity of Chol–MPB+ aggregates towards the CNT compared to the other Chol+ suspensions. Probably, during solubilization in water, the binding of Chol+ molecules with CNTs occur through the favorable interactions between the hydrophobic region of the cationic cholesterol with CNTs and the polar head groups remain exposed outside towards the bulk water. Size and solubility of CNT–Chol+ aggregates thus both depend upon the interaction between the CNTs and the Chol+ compounds.
We observed the CNTs in Chol+-wrapped aggregates after negative staining using uranyl acetate. In low magnification, representative formulation showed 'dendrite'-like structures with 'noodle'-like morphologies (figure 10(a)). Under high magnification, these appear as an array of small particles attached to some thread-like structures (figure 10(b)). The other formulations showed similar morphology as shown in figure 10. These results are in agreement with the suggestions put forward by Ciani et al from the calculations based on the density functional theory. These authors found that, considering the superposition of the cholesterol and CNT bands, cholesterol exerts little or no effect on the electrical properties of the CNT. However, inclusion of positive charge enhances the interaction of CNT with cholesterol and hydrophobic forces may be operational in aqueous suspensions [55].
After physical characterizations of CNT–Chol+ aggregates, we examined the effect of CNT incorporation on DNA binding (complexation) and the DNA release properties from the resulting complexes (dissociation). Towards this end, we performed an EB (ethidium bromide) exclusion assay and an EB re-intercalation assay. The EB exclusion assay (figure 11) indicated nearly comparable binding of the most Chol+ to DNA except CNT–Chol–PB+, which induced ∼80% EB release. In other words, ∼80% DNA binding was observed with CNT–Chol–PB+. The other Chol+ formulations showed ∼70% DNA binding. It suggests that electrostatic interactions between the CNT–Chol+ and DNA alone do not drive the complex formation. Additional factors also influence the binding of DNA with the CNT–Chol+ aggregates. Probably, it is the extent and the mode of wrapping of DNA (van der Waals interactions) on the CNT simultaneously decide the extent of exclusion of EB molecules from the CNT–Chol+–DNA complex and their re-intercalation to released DNA as well. Both available CNT surfaces and charge density on the Chol+–CNT aggregates may determine the interaction and the complex formation between the CNT with DNA [56].
Similarly, re-intercalation of EB into duplex DNA upon release of DNA from the CNT–Chol+–DNA complex is simultaneously decided by both Coulombic association of DNA on the CNT–Chol+ and wrapping of DNA by CNTs. The extent of re-intercalation of EB gives a measure of the extent of DNA release from the CNT–Chol+–DNA complexes. Greater extent of DNA release indicates that DNA binding to CNT–Chol+ aggregates is governed predominantly via electrostatic interactions and less via π–π stacking-based wrapping. The anionic SDS micelles added to CNT–Chol+–DNA complex (figure 11(B)) compete with the DNA for cationic cholesterol which is bound to the CNT–Chol+ complexes stabilized by the electrostatic force. Because of the electrostatic nature of such interactions, SDS-induced removal of Chol+ from such CNT–Chol+–DNA complexes do not significantly affect the wrapped DNA. This is the reason why each formulation could release a lesser amount of DNA from the CNT–Chol+–DNA complexes compared to Chol+–DNA 'cholaplexes' themselves. It further supports the notion of the dual mode of DNA binding with the CNT–Chol+ aggregates. Thus, the extent of DNA release from any CNT–Chol+ aggregate was determined by how much complexation contributed to electrostatic interactions and to what extent CNT surface wrapping by DNA is operational [57].
Blood serum is one of the many obstacles in the way of success for synthetic vector-mediated gene delivery. This is due to the fact that the serum proteins lead to the decomplexation of cationic cholesterol/DNA cholaplexes [58]. Competition for DNA in cationic lipid–DNA complexes is generally believed to be the reason behind the serum-mediated dissociation of the cationic lipid–DNA lipoplexes [59]. It is therefore necessary to develop formulations that have high stability and compatibility in serum media. Each CNT–Chol+–DNA complex here show significant serum stability compared to their counterpart suspensions devoid of CNTs. In other words, the incorporation of CNTs in cationic cholesterol suspensions increases its serum stability. Probably, it is due to the presence of CNTs in the formulation, the adsorption of serum proteins on CNT–Chol+–DNA complexes is impeded.
Further, cells were examined under confocal microscopy and analyzed by an image browser (figure 13). These images clearly show that, in the presence of CNTs, a significantly enhanced amount of anti-rabbit-FITC–Chol–DOB+ bind to the cell surface and internalize inside the cells. It also shows that incorporation of CNTs enhances the cell surface adherence and internalization of the Chol–DOB+aggregates. Therefore these CNT-loaded Chol–DOB+ suspensions may be useful for further biological studies.
5. Conclusions
In summary, we have demonstrated a simple scheme to disperse HIPCO single-wall carbon nanotubes in water. The cationic cholesterol compounds possessing ether linkage and the heterocyclic head groups were used for the CNT solubilization in water. All the four compounds used here disperse SWCNTs in water to afford stable CNT–Chol–MMB+, CNT–Chol–DOB+, CNT–Chol–PB+ and CNT–Chol–MPB+ suspensions. CNT loading in these suspensions were ∼100 times higher than that of the commonly used surfactants. Each suspension was then characterized by spectroscopic, DLS, zeta potential measurements, electron microscopic and other physical methods. These studies reveal the presence of highly segregated ('debundled') CNTs which remain dispersed with the help of cationic cholesterols in such suspensions. Formulations that contain CNTs show higher DNA binding, nearly comparable release and greater stability and compatibility in FBS compared to the Chol+ suspensions alone which did not contain any CNTs. Confocal studies confirm that CNT-based formulations adhere to HeLa cell surfaces and get internalized more efficiently than their counterpart formulations devoid of CNTs. Thus the incorporation of CNTs in these cationic cholesterol suspensions enhances their usefulness for biological studies. In addition, SWCNTs suspended in a significant concentration by this means can now be used for the creation of other novel composite materials, for the self-assembly of CNTs in suspension, and even for use as electrochemical sensors in water. Work is now underway to investigate these aspects.
Acknowledgments
This work was supported by a funding (J C Bose Fellowship to SB) from the Department of Science and Technology (DST). BSC thanks DST for a postdoctoral fellowship. For the SEM, TEM and DLS we thank INI and MBU, Indian Institute of Science, respectively.