Abstract
BaTi0.95Fe0.05O3−δ has been suggested to be an intrinsic dilute magnetic oxide with a clear dependence of magnetism on oxygen vacancy concentration. However, it has also been shown that the dopant Fe ions distribute themselves rather inhomogeneously within the lattice, though without disrupting the crystal phase of the parent BaTiO3. With the help of x-ray absorption spectroscopy (XAS), here we find that the incorporation of a larger amount of anion vacancy pushes this inhomogeneity to the extreme, leading to the precipitation of Fe metal clusters. It is also observed that the residual solid, without the Fe-metal cluster, undergoes massive structural and compositional reorganization.
Export citation and abstract BibTeX RIS
1. Introduction
Barium Titanate (BaTiO3), the prototype room-temperature ferroelectric material, remains today a subject of intense research. There have been sincere attempts to create multiferroicity by large doping of magnetic dopant ions in place of Ti in BaTiO3, and thereby creating high-temperature magnetic order too. However, even though a very large amount of doping of Fe in BaTiO3 seems possible (up to ∼84%) [1, 2], this doping induces a structural phase transition of BaTiO3 from the coveted pseudocubic (structure shown in figure 1(a)) to its hexagonal 6H polymorph (structure in figure 1(b)) only with a small doping (5–10%) [1–7]. The ferroelectricity in the 6H form is of a different kind, and unlike the pseudocubic form, the pure 6H BaTiO3 is ferroelectric only below 74 K [8].
In order to eliminate this problem and realize possible room temperature applications, thin films of doped BaTiO3 were also synthesized, with which it was possible to stabilize the pseudocubic structure of BaTiO3 even with a very large doping (>0.5) [9, 10], and possible multiferroicity was reported. More recently, there have been attempts to achieve the same result via interface interactions in artificial multiferroic tunnel junctions, consisting of a pure BaTiO3 layer sharing an atomically flat, defect-free interface with a metallic Fe layer, with more success [11–14].
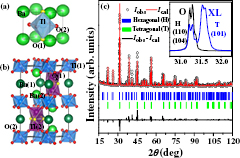
Figure 1. (a) Structure of cubic BTO, (b) 6H BaTiO3 structure and (c) powder XRD from RFBTO05XL (circles) along with the refined spectrum, shown on the data as a solid line. The inset shows the main intense peaks of RFBTO05XL (XL) and OFBTO05 (O).
Download figure:
Standard image High-resolution imageAn alternative proposal was put forward by us a few years ago [15], where it was suggested that the doping level be kept low, thereby keeping the structure and ferroelectricity minimally disturbed, in order to try to develop a dilute magnetic signal [16] in the bulk form of the material. Dilute magnetic oxides (DMOs) are generated in wide-band-gap oxide semiconductors by doping them with transition metal ions in very small quantity, which often exhibit a high-temperature ferromagnetic response, and therefore can provide spin-polarized current in a semiconductor device at or above room temperature. We argued that Fe-doped BaTiO3 could be one such member [15], for which the solubility of Fe-ions in BaTiO3 is very high i.e. uncertainty about 'intrinsic' doping is greatly reduced, and it is also rather easy to obtain the compounds in clean, single-crystalline form. However, growth of BaTiO3 single crystals in a floating zone furnace forces its 6H hexagonal polymorph (figure 1(b)) to stabilize even without doping. Although precipitation of no other crystalline phase could be detected in such Fe-doped BaTiO3 crystals possessing room-temperature ferromagnetism [15], an unusual preference was observed for the close proximity of dopant Fe-atoms within the structure (both the Ti2 sites (see figure 1(b)) of few specific Ti2O9 units are occupied by dopant Fe), instead of completely random distribution [15]. A similar trend towards lattice matched clustering has also been observed in other widely varying systems [17–19]. It therefore appeared that the doping of foreign ions inside a given lattice often occurs in an unexpected manner, giving rise to unusual properties like high-temperature ferromagnetism in DMO and naturally, theoretical models of dilute magnetism based on the random dopant distribution picture might be inadequate for describing such systems. In other words, it may not be technically correct to identify such magnetic signals as true dilute magnetic signals in a strict, conventional sense.
One critical confirmation of the intrinsic nature of 'dilute magnetism' reckons upon its dependence on associated defects [20, 21]. In the case of DMOs, this is manifested in its dependence on oxygen vacancies [22–25]. It has been shown in various reports that oxygen vacancies add carriers to the systems and enhance the ferromagnetic moment. Therefore, as a natural extension of our previous work [15], we have investigated the effect of mild variation in oxygen vacancy content (δ) on ferromagnetism [26] in BaTi0.95Fe0.05O3−δ. We indeed observed nearly proportional and reversible dependence of magnetic moment on δ [26]. However, x-ray absorption near edge spectroscopy (XANES) and extended x-ray absorption fine structure (EXAFS) studies revealed a further escalation of such lattice matched clustering, in which all the dopant Fe atoms almost exhaustively form Fe2  units within the 6H BaTiO3 structure having most of the oxygen vacancies being accumulated around the same [27]. It then becomes an interesting challenge to push the system even further and probe the evolution of the structure and properties of the material. Here, we report detailed macroscopic as well as microscopic studies on a new BaTi0.95Fe0.05O3−δ sample, where δ has been attempted to push to even higher values. Surprisingly, the sample showed a sudden disruption of the stability of 6H hexagonal structure, and a massive phase separation (nearly 70 : 30) into tetragonal and hexagonal BaTiO3 polymorphic structures was observed. We found that this increase in oxygen vacancy content δ destabilized the above-mentioned Fe2
units within the 6H BaTiO3 structure having most of the oxygen vacancies being accumulated around the same [27]. It then becomes an interesting challenge to push the system even further and probe the evolution of the structure and properties of the material. Here, we report detailed macroscopic as well as microscopic studies on a new BaTi0.95Fe0.05O3−δ sample, where δ has been attempted to push to even higher values. Surprisingly, the sample showed a sudden disruption of the stability of 6H hexagonal structure, and a massive phase separation (nearly 70 : 30) into tetragonal and hexagonal BaTiO3 polymorphic structures was observed. We found that this increase in oxygen vacancy content δ destabilized the above-mentioned Fe2  units, leading to precipitation of pure Fe metal clusters and a consequent large spurious magnetic moment. We therefore conclude that there is a general trend towards inhomogeneous placement of magnetic dopants inside the host oxide lattice, while maintaining the same crystalline phase, which, if pushed to an extreme, could give rise to metal cluster precipitation and extrinsic impurity magnetism. Until this happens, however, the dilute magnetic signal from a system should be accepted as an intrinsic one, though of course there is only a fine line between a true dilute magnetic signal and a signal of extrinsic origin (a signal from a precipitated Fe-metal cluster).
units, leading to precipitation of pure Fe metal clusters and a consequent large spurious magnetic moment. We therefore conclude that there is a general trend towards inhomogeneous placement of magnetic dopants inside the host oxide lattice, while maintaining the same crystalline phase, which, if pushed to an extreme, could give rise to metal cluster precipitation and extrinsic impurity magnetism. Until this happens, however, the dilute magnetic signal from a system should be accepted as an intrinsic one, though of course there is only a fine line between a true dilute magnetic signal and a signal of extrinsic origin (a signal from a precipitated Fe-metal cluster).
During the course of our extended study of dilute magnetism in Fe-doped BaTiO3 single crystal, it turned out to be extremely difficult to search for any small amounts of precipitated magnetic phases using conventional tools [28–33], because they are expected to be buried deep within the enormous volume of the 'nonmagnetic' bulk matrix. In fact, in the present case, simple lab-based techniques failed to indicate Fe-metal precipitation even though significant changes in overall crystal and chemical compositions were revealed. In such cases, it is much more sensible to apply element-specific experimental tools for the purpose of probing the changes carried out by the dopant ions, because it becomes possible then to selectively investigate only these ions while completely ignoring the background phase(s). Furthermore, it can be expected that the best-suited process would be to selectively choose the dopant atoms and extract the structural information around them, because crystalline structure is the most basic parameter associated with a solid phase, and therefore might be best used for the identification of the secondary phase, if any. Accordingly, over the last few years local structural probing techniques such as XANES and EXAFS have been utilized very efficiently to address this problem and clear up many confusions [34–39]. Here, we show that Fe K-edge EXAFS can successfully detect the presence of Fe metal clusters almost as directly as x-ray diffraction (XRD) identifies large quantities of secondary crystalline phases. This underlines the importance of EXAFS in investigating DMO materials.
2. Experimental methods
BaTi0.95Fe0.05O3−δ single crystal has been grown in a conventional floating-zone furnace [15]. The synthesis details of the nearly oxygen stoichiometric OFBTO05 and reduced RFBTO05M and RFBTO05L samples are given in [26]. Another new reduced sample was also made in the same way, but by making the crystal pieces much smaller in size to expose more surface area and thereby introducing an even larger amount of oxygen vacancies. This sample is the central point of this paper, and will be referred to below as RFBTO05XL. Powder XRD was carried out in a Bruker D8 Advance x-ray diffractometer using Cu Kα radiation. The diffraction data were fitted using the Rietveld method, and the refinement of crystal structure was carried out using FULLPROF [40]. The dc magnetization measurements were performed in a quantum design SQUID magnetometer. The EPR spectra were recorded at 77 K using an X-band (9.2 GHz) JEOL JES-FA200 ESR spectrometer. XAS experiments were carried out at the Elettra (Trieste, Italy) synchrotron radiation facility (11.1-XAFS beamline, exp. code: 20105245) in transmission geometry, at liquid-nitrogen temperature [41]. The details of the XAFS data analysis can be found in [27]. Further specific fitting details for this sample are given in the EXAFS results section.
3. Results and discussion
Figure 1 shows the XRD pattern of a RFBTO05XL sample along with the Bragg reflection from pure hexagonal (JCPDS No-01-034-0129) and tetragonal BaTiO3 (JCPDS No-01-074-1957). The refined pattern is plotted as a solid line over the experimental data points. In the inset, the maximum intense peak of RFBTO05XL is shown, along with the same for OFBTO05.
The double peaks at 2θ = 31.231, 31.309 match with the (1 1 0) and (1 0 4) peaks of the 6H structure, while the peak at 2θ = 31.542 provides a good match with the (1 0 1) peak of the tetragonal BaTiO3 structure. So, it is evident from these peak intensities that, in RFBTO05XL, a larger amount of tetragonal phase develops at the expense of the hexagonal phase. The hexagonal phase could be fitted well within the P63/mmc space group, while the tetragonal phase was fitted using the P4mm space group. The refinement suggested a combination of 30% hexagonal and 70% tetragonal phases in this sample, while no other impurity phase has been observed. By carefully measuring weight changes after each reduction process, δ ∼ 0.1 could be confirmed. Any further structural study using single crystal diffraction was prohibited due to large phase mixing.
Results of the magnetization experiments are summarized in figure 2. Panel (a) shows the magnetic field dependence of the magnetization of the RFBTO05XL sample at 300 K. For comparison, the same for the OFBTO05 and RFBTO05L samples [26] are also plotted in the panel. Like the other two, RFBTO05XL also shows ferromagnetism at 300 K, with clear hysteresis (see the inset of figure 2(a)), and a distinct tendency towards saturation at 1 Tesla magnetic field. Most importantly, the ferromagnetic moment shows a straightforward proportional dependence on oxygen vacancy concentration, with the RFBTO05XL moment being nearly 10 times larger than the same from OFBTO05. Figure 2(b) shows the temperature dependence of the magnetic susceptibility of the RFBTO05XL sample. The zero-field-cooled (ZFC) and Field-Cooled (FC) magnetization data have been collected under an applied field of 200 Oe magnetic field. Unlike OFBTO05 and RFBTO05L [26], the large divergence between the FC and ZFC curves of RFBTO05XL indicates the presence of magnetic metastability in this system. Although, in case of dilute magnetic systems, such divergences are often correlated with spurious superparamagnetic clusters [42] that might be present in the system, our preliminary characterization of the sample failed to confirm the presence of any such extrinsic phase(s). Another point to be noted is that, even though the M(T) curves resembled the mean field paramagnetic susceptibility curve, the inverse susceptibility (see inset of figure 2(b)) strongly refutes such a possibility. However, a contradictory result was obtained from the X-band EPR measurements. Figure 2(c) shows the X-band EPR measurements from the RFBTO05XL sample. The EPR spectra from OFBTO05 and RFBTO05L have already been presented and discussed in [26]. The EPR signal from the RFBTO05XL sample shows significant divergence from the other two, while its striking resemblance with a tetragonal BaTi0.98Fe0.02O3, possessing isolated Fe3+ spins in a tetragonal environment, could not be missed [43]. We can therefore conclude that the tetragonal component of the RFBTO05XL sample contains isolated, noninteracting Fe3+ ions (responsible for the observed EPR signal) with minimal oxygen vacancy, while there might be EPR inactive Fe ions (Fe2+: EPR inactive in microwave or X-band region) as well as large oxygen vacancies within the 6H hexagonal network, where accommodating a larger quantity of anion vacancies is energetically more favourable. However, this description fails to explain the observed large moment, because, in this case, at least some of the Fe ions within the major tetragonal phase would be noncontributing.
Figure 2. (a) The M(H) curves from OFBTO05 (solid line), RFBTO05L (dash line), RFBTO05XL (dotted line) samples at 300 K. Inset shows the coercivities. (b) The FC and ZFC magnetic susceptibilities for the RFBTO05XL sample, along with the χ−1(T) plot (see inset). (c) EPR spectra for RFBTO05XL, collected at 77 K, and the BaTi0.98Fe0.02O3 spectrum. (Reproduced from [43] with permission from IOP Publishing, copyright 2008).
Download figure:
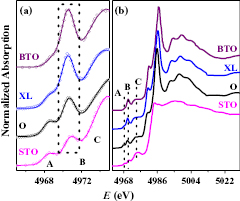
Standard image High-resolution imageTherefore, in order to understand the system properly, obtaining microstructural and electronic information from local probes like XANES and EXAFS appeared essential. The Ti K-edge XANES spectrum of RFBTO05XL (XL) and OFBTO05 (O) samples are shown in figure 3, and compared with the spectra of SrTiO3 (STO) and tetragonal BaTiO3 (BTO). In figure 3(a) the fitted pre-edge peaks A, B and C [27] are shown, indicating a significant intensity of the B peak in the RFBTO05XL sample, comparable to BTO. This shows that the oxygen vacancies do affect the local environment of Ti in RFBTO05XL, and bring forth non-centrosymmetric distortions [27] in the Ti-O network. The existence of a large quantity of tetragonal BaTiO3, like the phase in RFBTO05XL, explains this special feature of the Ti XANES pre-edge structure.
Figure 3. (a) Pre-edge region of the Ti K-edge XANES spectra from BTO, XL, O and STO. (b) Full-range normalized Ti K-edge XANES spectra.
Download figure:
Standard image High-resolution imageHowever the effect on Ti local structure and electronic state is weak compared with that around Fe ions (figure 4). The precise calibration of the energy scale was achieved by simultaneously measuring the absorption spectra of Fe metal foil placed after the sample. The first point is that the Fe4+ dopant oxidation state, which implies a high energy shift in Fe K-edge XANES [44], can be immediately excluded in this sample. It is known that, in BaTi1−xFexO3−δ solid solution, the Fe ions are mainly incorporated as Fe3+ in a low doping regime, and the progressive substitution of Ti4+ by Fe3+ is accompanied by the formation of oxygen vacancies in the hexagonal stacking layers of the 6H structure [2, 15]. However, only a very high doping of iron (x > 0.67) into the 6H structure at high temperature allows substitution of Ti4+ by Fe4+ ions [2]. Further, in our previous work [27], we have compared the experimental Fe K-edge XANES of oxygen-annealed BaTi0.95Fe0.05O3 (OFBTO05) and low-oxygen-vacancy doped BaTi0.95Fe0.05O3−δ (RFBTO05L) samples with those of Fe2O3 and FeO reference compounds, which demonstrated that even a small number of oxygen vacancies provoked a sudden shift of the Fe edge, signalling a reduction in the Fe oxidation state. The inspection of the pre-edge features demonstrated that the Fe ions adopted mainly an Fe3+ oxidation state in OFBTO05, and then changed to Fe2+ in the RFBTO05L sample. Interestingly, in the case of RFBTO05XL, the main effect of raising oxygen vacancies on the Fe K-edge XAS data reveals a further intriguing detail. In addition to the main edge position being matched with a Fe2+ species, there is also a significant raising of the pre-edge (7715 eV; figure 4(a)), which suggests the presence of a combination of different Fe valence states between 0 to 2+ in the material. In order to prove the hypothesis that there is a different structural environment for Fe ions, we have attempted to fit the normalized experimental Fe K-edge spectrum of RFBTO05XL as a linear combination of normalized RFBTO05L and Fe metal experimental spectra, and the best fit curve is shown in figure 4(b). The strong agreement between the data and the fit warrants definitive confidence in the model. The possibility of including FeO or Fe2O3 contributions to the linear combination can be excluded. The best fit reveals that there might be approximately 22% Fe ions present as part of a metal cluster in RFBTO05XL. This behaviour is not surprising, as phase separation due to the solubility limit in vacancy-doped compounds has been verified in other systems as well [45]. However, it appears that, when pushed to the extreme, the lattice matched clusters of OFBTO05 and RFBTO05L [15, 27] degrade, leading to the precipitation of pure Fe metal.
Figure 4. (a) Pre-edge region of Fe K-edge, magnified (2.5 times) for the sake of clarity. (b) Close view of the experimental Fe K-edge XANES spectra, along with the representative of different Fe valence state: Fe (Fe0), FeO (Fe2+), Fe2O3 (Fe3+). The lower curves show the fitting of RFBTO05XL XANES data (symbols) as a linear combination of RFBTO05L and Fe metal spectra.
Download figure:
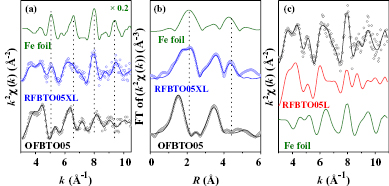
Standard image High-resolution imageA clearer picture of the local structure is obtained from the analysis of the Fe EXAFS spectrum. Figures 5(a) and (b) show the experimental EXAFS data and its Fourier transform, along with the respective best-fit spectra of the RFBTO05XL and OFBTO05 samples and the same for Fe metal foil. Interestingly, in the extended region the EXAFS spectrum of RFBTO05XL differed significantly from that of OFBTO05, which required additional analytical considerations. From the Fourier transform (figure 5(b)), it is evident that oxygen reduction provokes large structural effects: the first peak (around 1.5 Å) clearly presents a bimodal distribution, and additional peaks become evident around 3 Å and 4.5 Å. Based on the raw EXAFS data, these features can be immediately related to the presence of Fe metal phase. In fact the spectral features evident at 5, 6.5, 8 and 9.5 Å−1 in the RFBTO05XL spectrum, but not in the OFBTO05 one, can be directly associated to those observed in the Fe metal EXAFS spectrum (see the figure in Supporting Information (stacks.iop.org/J.PhysCM/26/196001/mmedia)). This finding further confirms the segregation of Fe metallic regions in the RFBTO05XL sample. Here the chemical selectivity of EXAFS works efficiently to detect segregated Fe phases that are hardly visible in the diffraction patterns due to their weaker contribution, and thereby confirms the utility of the EXAFS technique in probing DMS systems. An attempt to fit the RFBTO05XL Fe EXAFS spectrum using the same structure as that of RFBTO05L failed, but the fitting definitively improved when two possible phases for Fe were assumed: the RFBTO05L and metal Fe phases (figure 5(c)), consistent with the preliminary results from XANES fitting. The necessity of including the two phases for Fe ions for quantitative analysis raises the number of structural parameters. We briefly state the constraints adopted to reduce the free parameters and achieve reliable structural results. The Fe local structure in the RFBTO05L phase has been modelled according to the picture described earlier, i.e. Fe located at the Ti2 sites [27] with coordination numbers lower than 6 due to oxygen deficiency. The reduced coordination has been weighted in the total fitting as (1 − x). After preliminary attempts, we improved the best fit by fixing the NFe−O = 5(1 − x), which confirmed that the oxygen vacancies are mainly localized around Fe ions in RFBTO05L [27]. In the Fe metal phase the shell distances were constrained to the Fe-bcc structure:  , RFe−Fe2 = abcc,
, RFe−Fe2 = abcc,  , and
, and  , so that only the Fe-bcc lattice parameter (abcc) was refined. In addition, the coordination numbers were kept fixed to the Fe-bcc bulk values and weighted by the free parameter x, equating to the fraction of the Fe metallic phase: NFe−Fe1 = 8x, NFe−Fe2 = 6x, NFe−Fe3 = 12x, NFe−Fe4 = 24x. A fifth Fe–Fe shell was found in the Fe-bcc structure, with RFe−Fe5 =abcc
, so that only the Fe-bcc lattice parameter (abcc) was refined. In addition, the coordination numbers were kept fixed to the Fe-bcc bulk values and weighted by the free parameter x, equating to the fraction of the Fe metallic phase: NFe−Fe1 = 8x, NFe−Fe2 = 6x, NFe−Fe3 = 12x, NFe−Fe4 = 24x. A fifth Fe–Fe shell was found in the Fe-bcc structure, with RFe−Fe5 =abcc . This contribution was expected to provide a relatively intense EXAFS signal due to the multiple scattering contribution of Fe–Fe–Fe atoms aligned along the bcc cube diagonal. As described in [27], new contributions are included in the fitting only if they statistically improve it. In this case, single and multiple scattering (triple and quadruple) scattering terms of the Fe–Fe5 shell did not lead to any statistical improvement. We must stress that all of the first 4 shells of Fe-bcc structure are correctly reproduced, having structural parameters (Ri and Ni) constrained to the Fe-bcc bulk structure, and therefore the EXAFS and XANES data coherently support the hypothesis of about 20% of Fe atoms segregated in a metallic phase. All the extracted lattice information from the analysis of EXAFS data have been summarized in table 1. There are several possible explanations for the weakness of Fe–Fe5 in our data: it may be due to the structural disorder in the Fe-rich phase, which has a larger effect on the multiple scattering contributions. Or it may be an effect of the small size of Fe-bcc phase particles, as in clusters their high surface-to-volume ratio may reduce the observed coordination numbers [46], more for the greater distances. In our data, due to the relatively short k-range and the number of structural parameters involved, it is impossible to reliably take into account such effects, which can get partially masked by apparently larger disorder factors. We are unable to asses the average size of Fe clusters. In fact, the overall amount of the Fe-bcc phase is definitively weak (0.05 × 0.2), and even in the case of large clusters (i.e. several nm) they will be hardly detectable by XRD. The evidence of Fe-bcc contributions up to the fourth shell may establish an average cluster size larger than 1–2 nm. In our previous work [27], the Fe–Fe distance (RFe−Fe) in the moderately doped BaTi0.95Fe0.05O3−δ was found to be around 2.47Å, definitively similar to the nearest-neighbour distance of Fe-bcc (RFe−Fe1). In this case we maintained fixed conditions of RFe−Fe = RFe−Fe1 and
. This contribution was expected to provide a relatively intense EXAFS signal due to the multiple scattering contribution of Fe–Fe–Fe atoms aligned along the bcc cube diagonal. As described in [27], new contributions are included in the fitting only if they statistically improve it. In this case, single and multiple scattering (triple and quadruple) scattering terms of the Fe–Fe5 shell did not lead to any statistical improvement. We must stress that all of the first 4 shells of Fe-bcc structure are correctly reproduced, having structural parameters (Ri and Ni) constrained to the Fe-bcc bulk structure, and therefore the EXAFS and XANES data coherently support the hypothesis of about 20% of Fe atoms segregated in a metallic phase. All the extracted lattice information from the analysis of EXAFS data have been summarized in table 1. There are several possible explanations for the weakness of Fe–Fe5 in our data: it may be due to the structural disorder in the Fe-rich phase, which has a larger effect on the multiple scattering contributions. Or it may be an effect of the small size of Fe-bcc phase particles, as in clusters their high surface-to-volume ratio may reduce the observed coordination numbers [46], more for the greater distances. In our data, due to the relatively short k-range and the number of structural parameters involved, it is impossible to reliably take into account such effects, which can get partially masked by apparently larger disorder factors. We are unable to asses the average size of Fe clusters. In fact, the overall amount of the Fe-bcc phase is definitively weak (0.05 × 0.2), and even in the case of large clusters (i.e. several nm) they will be hardly detectable by XRD. The evidence of Fe-bcc contributions up to the fourth shell may establish an average cluster size larger than 1–2 nm. In our previous work [27], the Fe–Fe distance (RFe−Fe) in the moderately doped BaTi0.95Fe0.05O3−δ was found to be around 2.47Å, definitively similar to the nearest-neighbour distance of Fe-bcc (RFe−Fe1). In this case we maintained fixed conditions of RFe−Fe = RFe−Fe1 and  =
=  .
.
Figure 5. (a) k2 weighted Fe K-edge experimental (open circles) and best fit (full lines) EXAFS spectra of OFBTO05, RFBTO05XL and Fe metal. (b) The Fourier transform moduli of experimental (symbols) and best fit curves (full lines). (c) k2 weighted Fe K-edge experimental (dots) and best fit (full lines) EXAFS spectra of RFBTO05XL, along with the same for RFBTO05L and Fe-metal, which were the two components of the fitting.
Download figure:
Standard image High-resolution imageTable 1. Results of RFBTO05XL Fe K-edge EXAFS data analysis [47]. Physical constraints were imposed among the parameters to reduce the correlation among them (details in [27]). The refined fraction of the Fe metal (Fe-BTO) phase is around 21% (79%). The standard uncertainty in the last digit of refined parameters are in parentheses.
| Fe-BTO | Fe cluster | ||||||
|---|---|---|---|---|---|---|---|
| Shell | N | R (Å) | σ2 (×102 Å2) | Shell | N | R (Å) | σ2 (×102 Å2) |
| Fe–O | 5 | 1.99(2) | 0.98(9) | Fe–Fe1 | 8 | 2.49 | 0.30(2) |
| Fe–Fe | 1 | 2.49 | 1.0 | Fe–Fe2 = abcc | 6 | 2.87(1) | 1.0(1) |
| Fe–Ba | 8 | 3.46(2) | 1.8(4) | Fe–Fe3 | 12 | 4.06 | 1.0(2) |
| FeOTi | 3 | 4.01(3) | 1.7(5) | Fe–Fe4 | 24 | 4.76 | 1.8(4) |
| FeOBa | 6 | 4.77(5) | 1.4(4) | ||||
| 1 − x | 0.79 | x | 0.21(1) | ||||
Now it is qualitatively possible to correlate the observed structural and compositional reorganizations with increasing oxygen vacancy. As noted above, there is a presence of lattice matched clusters or Fe2 -like dimers already in RFBTO05L, and even to some extent in OFBTO05 [15, 27]. It appears that, when the increased oxygen-vacancy concentration goes beyond the energetically permissible limit, such inhomogeneities degrade further, and the system throws a significant amount of Fe2+-
-like dimers already in RFBTO05L, and even to some extent in OFBTO05 [15, 27]. It appears that, when the increased oxygen-vacancy concentration goes beyond the energetically permissible limit, such inhomogeneities degrade further, and the system throws a significant amount of Fe2+-  complex out of the lattice as a pure Fe metal cluster. Consequently, the resulting low-Fe-doped BaTiO3 creates a mixed structural environment with various Fe-dopant distributions, consistent with the literature [4, 48, 49]. The EPR result suggests that the tetragonal phase possesses only a small percentage of magnetically uncorrelated Fe3+, and consequently less vacancy, while apparently the majority of Fe2+ and
complex out of the lattice as a pure Fe metal cluster. Consequently, the resulting low-Fe-doped BaTiO3 creates a mixed structural environment with various Fe-dopant distributions, consistent with the literature [4, 48, 49]. The EPR result suggests that the tetragonal phase possesses only a small percentage of magnetically uncorrelated Fe3+, and consequently less vacancy, while apparently the majority of Fe2+ and  resides within the hexagonal environment. The much-enhanced ferromagnetic signal is expected to arise mainly from the precipitated Fe metal clusters, which remained invisible under standard experimental tools. Comparing the coercivity and the shape of the M(H) curve with the standard literature [50], the presence of Fe nanoclusters of around 10 nm in the sample can be estimated. Overall, our results establish that, in the absence of careful microscopic studies, it is prohibitively difficult to reach a conclusion about the intrinsic magnetism of dilute doped systems.
resides within the hexagonal environment. The much-enhanced ferromagnetic signal is expected to arise mainly from the precipitated Fe metal clusters, which remained invisible under standard experimental tools. Comparing the coercivity and the shape of the M(H) curve with the standard literature [50], the presence of Fe nanoclusters of around 10 nm in the sample can be estimated. Overall, our results establish that, in the absence of careful microscopic studies, it is prohibitively difficult to reach a conclusion about the intrinsic magnetism of dilute doped systems.
Lastly, it is worth mentioning the effect of such fluctuations on the dielectric constant and ferroelectric properties of these materials. It was observed earlier that the ferroelectricity becomes completely lost when BaTiO3 is doped with only 1% Fe or more in hexagonal structure [15, 26]. Moreover, the presence of a larger concentration of the carrier makes the system substantially conducting, thereby dilapidating the existence of any ferroelectricity in BaTi0.95Fe0.05O3−δ [26]. But the situation becomes somewhat different in the present case of RFBTO05XL, where a larger part of the system (∼ 70%) is tetragonal, with very little noninteracting dopant Fe3+, and plausibly a small amount of oxygen vacancy, and this part might therefore be expected to exhibit ferroelectricity. This situation is similar to the case of diffuse phase mixture in doped BaTiO3 [51, 52], where very small hexagonal regions exist within a larger tetragonal matrix. Recently, we have shown [53] in a Ca, Fe co-doped BaTiO3 system that hexagonal microregions do form inside an overwhelmingly tetragonal structure, where XRD data clearly show the presence of a pseudocubic structure. Interestingly, the ferroelectricity is lost even in this system when oxygen vacancies are poured into these tiny hexagonal patches (figure 2 of [53]). Therefore, it can be concluded that ferroelectricity from the tetragonal regions, if present at all, will definitely be masked in the presence of much larger hexagonal regions with larger oxygen vacancies, along with few metallic Fe-clusters, in RFBTO05XL.
4. Conclusions
In summary, detailed structural and physical property measurements have been carried out on 5% Fe-doped BaTiO3 single crystals with strong oxygen vacancy. Earlier studies of the same system with much lower vacancy concentration already indicated a somewhat inhomogeneous distribution of Fe-dopants within the lattice, which took an extreme turn in the presence of larger vacancies, and as a result nearly 30% Fe atoms come out of the system as an Fe metal cluster. The rest of the system reorganizes itself as oxygen deficient and low Fe doped (∼ 3% Fe doped) BaTiO3, which creates a mixture of hexagonal and tetragonal structural regions. Evidently, the real cause of this process remained undetectable under the resolution level of the XRD or other standard probes, and became visible only with the use of element-specific local structural probes like XANES and EXAFS, thus establishing their utility in this context once again.
Acknowledgments
SR acknowledges CSIR, India for funding. Experiments at the Elettra-Sincrotrone Trieste have been supported partially by ICTP (exp. 20120265) and partially by the Italian Government funded user program.