Abstract
High-performance Fe-sheathed MgB2 monocore wires have been fabricated by an in situ method using cheap oleic acid additions as a reliable C source for B lattice substitution. We have analysed the cases of soaking B or Mg + B powders in oleic acid and the influence of the heat treatments used at different steps of wire manufacturing on their superconducting properties. X-ray diffraction, electronic microscopy, and thermogravimetric analysis, together with magnetic and electric transport measurements, have been used to characterize the wires. Doping with oleic acid considerably improves the critical current density, Jc, at medium and high magnetic fields at 5 and 20 K, without deterioration of Jc at low magnetic fields.
Export citation and abstract BibTeX RIS
1. Introduction
Metal/MgB2 composite wires and tapes are good candidates for practical large-scale superconducting applications at operation temperatures in the range 20–30 K. Long-length conductors with high critical current density, Jc, were first fabricated by the powder-in-tube (PIT) technology [1–6] using in situ and ex situ procedures and various metal cladding to avoid reactivity with MgB2 constituents. Attempts to reduce the high porosity inherent to PIT in situ wires, or to improve the grain connectivity on PIT ex situ conductors [7, 8], have been followed by different processing routes. One to be noted is internal Mg diffusion (IMD) [9], which, with in situ reactions, produces a hollow cylinder of highly dense MgB2 with high Jc-values [10, 11].
Improvements in Jc performance at high fields are also needed for many applications [7]. Doping with different elements and compounds was intended to enhance the flux pinning of pure MgB2 materials without deterioration of other relevant superconducting properties. By grain refinement and by the addition of carbon, or carbon-containing compounds, significant enhancement of the superconducting properties has been obtained [8, 12]. Ternary Mg(B1−xCx)2 compounds are isostructural in a wide range of compositions, with carbon randomly substituting boron in the MgB2 lattice. This causes a reduction of the unit cell a-parameter, while the c-axis remains constant, and the Hc2 anisotropy and critical temperature, Tc, decrease. Moreover, the irreversibility line Hirr,(T), the magnetic flux pinning, and Hc2(0) increase with C substitution [13, 14]. Consequently, carbon chemical doping of MgB2 has been widely performed, and it is a very effective way to improve the Jc(H) performance at low temperatures.
On composite wires and tapes, using both pre-reacted MgB2 and unreacted Mg and B, C doping has been obtained with many carbon sources [12]. Among inorganic compounds, the addition of SiC nanoparticles [15], graphite powders [16, 17], nanoscale diamond powders [17], carbon nanotubes [18], or carbon nanoparticles [19], induces the partial substitution of B by C, and it is compatible with long-length wire fabrication procedures.
Organic liquids such as acetone, ethanol, and toluene have been introduced to help the homogeneity of Mg and B mixtures during wet ball milling [20], being removed before wire manufacturing. This avoids particle agglomerations and produces materials with smaller grain sizes and higher Jc-values, but also may induce C doping. Fe-sheathed wires made with B and MgH2 powders mixed with aromatic hydrocarbons (benzene, naphthalene, and thiophene) were reported [21] to produce effective C doping and Jc improvements. From the shift of characteristic x-ray diffraction (XRD) peaks, the substitution of B lattice sites by C atoms, x, is estimated to be <0.015, at least one order of magnitude lower than the total amount of C added to the precursors. Moreover, it was realized that mixtures of B powders with malic acid dissolved in toluene after vacuum drying help to form homogeneous C coatings of B grains. Those treated grains mixed with Mg powders yield materials with improved Jc and substitutions x < 0.035, much smaller that the initial C provided in the precursor [22]. Hydrocarbons, carboxylic acids, carbohydrates, alcohols, ketones, amines, etc, in the form of powders, liquids, or solutions, have been used as C sources for B + Mg or MgB2 powders [12]. However, there is little knowledge of the substitution mechanisms, the chemical processes involved, and the carbonaceous secondary phases created in each case.
A common feature of successful doping with C-rich organic sources is the production of highly reactive C sources at the atomic scale at temperatures below the formation temperature of MgB2 (>580 °C). Therefore, it is possible to decrease the annealing temperatures needed for effective C doping and obtain higher carbon substitution [12]. This will result in larger lattice disorder and finer grains that would increase the magnetic flux pinning, Hc2, and raise the irreversibility line.
Considering carboxylic acids as the C source of MgB2 composite wires or tapes, there are also chemical interactions with secondary phases present in Mg, B, or MgB2 components, such as MgO or B2O3. These effects are more important for acetic [23] or malic [22, 23] acids, which have short aliphatic C- chains with C versus O ratios r ≤ 1, and stronger acidity than stearic acid [24] with longer chains, r = 9.
The effect of oleic acid (C18H34O2, r = 9) addition on MgB2 wires has not been reported so far, but previous results of Devener et al [25] have shown that oleic acid binds efficiently to fresh unoxidized boron surfaces via B–O–C bond formation, and that it provides an effective barrier to air oxidation. It is also known that oleic acid surfactant coatings provide chemical stability to magnetic nanoparticles such as Co, Ni, Fe, and FePt [26], which easily oxidize. It is very likely that similar effects would operate for Mg fine powders. Consequently, doping with oleic acid would fulfil the advantages of the best organic liquids in the C substitution of B on PIT composite MgB2 wires and may prevent further oxidation during fabrication.
In this contribution, we report on the addition of oleic acid as the C source for B substitution on monocore MgB2 wires fabricated by the PIT method with Fe sheaths and in situ reaction of the components. Doping with oleic acid is worth researching since it is a liquid at room temperature (melting point 13 ° C), it may prevent oxidation, and it has good lubricant properties, low price and relatively high C/O ratio. In particular, the effects of oleic acid on B, Mg, and B + Mg powders during thermal annealing have been studied. The microstructure, phase composition, and superconducting properties of these doped wires have been analysed and compared with those of undoped wires.
2. Experimental procedures
2.1. Preparation of wires
Monofilament conductors have been fabricated by the PIT method using in situ reactions of the components and mechanical conformation by drawing through round dies. Fe tubes (99.5%, Goodfellow, with outer diameter 5 mm and 0.25 mm wall thickness) were filled by hand with different precursor powder mixtures (details are given below). During drawing, an intermediate annealing was necessary to reduce the Fe sheath's work hardening. The final wires have 1.1 mm outer diameter.
The starting materials were commercial Mg powders (99.8%, maximum particle size 250 μm, Goodfellow), amorphous B powders (99% and mean particle size lower than 1 μm, New Metal & Chemicals Ltd) with stoichiometric proportions, and liquid oleic acid (99%, Alfa Aesar). All the precursors were mixed for 30 min using a Retsch MM 2000 mixer mill. Results on four different wires are reported.
- W0 (Undoped reference wire). This was made from a dry mixture of Mg and B powders filled into Fe tubes. An intermediate heat treatment at 550 ° C × 0.5 h in Ar atmosphere was done during drawing.
- W5-n (Mg + B powders mixed with oleic acid, non-preheated). Oleic acid (5 wt% of total MgB2) was mixed with Mg and B powders, and the obtained mixture directly filled into the Fe tubes without preheat treatments, thus keeping inside the full oleic amount. The intermediate annealing during drawing was done at 320 ° C × 24 h (below the boiling point of oleic acid, 360 ° C) in Ar atmosphere.
- W10 (Mg + B powders mixed with oleic acid and preheated). Oleic acid (10 wt% of total MgB2) was mixed with Mg and B powders, and before filling the Fe tube the mixture was preheated at 400 ° C × 1 h in Ar to evaporate the less-bounded and unreacted molecules, i.e. to eliminate the oleic acid excess. Two wires with different intermediate softening annealing treatments during drawing were prepared: 550 ° C × 0.5 h and 400 ° C × 0.5 h, in Ar atmosphere.
- W10-B (B powders treated with oleic acid and preheated before Mg mixtures). First, the B powders were mixed with oleic acid (10 wt% of total MgB2) and heat treated at 500 ° C × 1 h in vacuum (0.1 mbar) or Ar, to evaporate the oleic acid excess. The resulting powders were mixed with Mg and used to fill the Fe tubes. An intermediate annealing for Fe softening at 550 ° C × 0.5 h in Ar atmosphere was done during drawing.
Two different final annealing conditions to form the superconducting phase have been studied: 700 ° C × 2 h and 670 ° C × 5 h, in vacuum (0.1 mbar).
2.2. Physical characterization
The phase composition and microstructure of the wires was analysed by x-ray diffraction (XRD, RIGAKU D/max 2500 Cu Kα), field-emission scanning electron microscopy (FESEM) using angle-selective backscattered electrons (AsB), which gives information about the phase composition and crystal orientation, and energy-dispersive x-ray spectroscopy (EDX).
Thermogravimetric analysis (TGA) was performed using a TA Instruments SDT Q600 system to register the weight loss along programmed temperature scans of oleic acid and mixtures of oleic acid with Mg, B, and Mg + B powder samples. Pt crucibles and a heating ramp of 20 °C min−1 from room temperature up to 550 ° C in Ar flow were used.
The magnetic measurements were carried out on 5 mm long cylindrical samples using a superconducting quantum interference device (SQUID, Quantum Design MPMS-5T) and a physical properties measurement system (Quantum Design PPMS-9T and PPMS-14T) with the field perpendicular to the sample axis and zero-field cooling. The ac magnetic susceptibility χac (in-phase, χ', and out-of-phase, χ'', components) was measured as a function of temperature at a frequency of 120 Hz and magnetic field amplitude of 0.1 mT. Magnetization measurements were done at 5 and 20 K. To avoid the contribution of the Fe sheath to the magnetic measurements, it was removed by careful mechanical polishing.
Electric transport measurements were performed inside the bore of an 8 T coil magnet immersed in liquid helium using wire sample lengths of 3.7 cm, maximum currents about 220 A, and magnetic fields perpendicular to the wire. In order to avoid the heating of the sample, current pulses of 2 s were applied to measure the voltage versus current curves, V(I). A four-point technique was used, and the critical current was estimated by the usual 1 μV cm−1 criterion.
3. Results and discussion
3.1. Structure, C doping, and microstructure of wires
The XRD patterns obtained on wire cores, after removing the Fe sheath, show in all wires the characteristic peaks of the MgB2 main phase together with small amounts of MgO and without signatures of other crystalline compounds. In figure 1, the characteristic Bragg reflexion peaks (002) and (110) of the MgB2 structure are displayed. It is observed that the position of the (001) peaks remains invariant for all wires while the (110) peak moves towards higher 2θ angles for the doped wires. This indicates that the doping has not affected the c-axis cell parameter whereas the a-axis length decreases (see table 1), which confirms the random substitution of B by C in the MgB2 lattice [13]. The averaged doping amount, x, of the Mg(B1−xCx)2 grains in the wire core have been obtained from the estimated a unit-cell parameter and by using the nearly lineal dependence a(x) observed by Kazakov et al [27] for doped single crystals. The error in the estimation of carbon doping is about 15–20%; therefore, the x-value of wire W10 would be higher but close to the W10-B value. In the figure it is also observed that the height of the MgO peak is similar for all samples, although it is slightly higher for W5-n and lower for W0.
Figure 1. Detail of the x-ray diffraction patterns of the wire's superconducting cores at angles around the (002), (110), and (102) MgB2 peaks. The (220) peak of MgO is also shown.
Download figure:
Standard image High-resolution imageTable 1. Lattice parameters obtained from Rietveld refinements and FWHM of the (110) peak deduced from XRD measurements of core wires. Critical temperatures Tc and transition widths ΔTw obtained from χac. Average substitution of B by C in Mg(B1−xCx)2 grains estimated from the a(x) variation according to [27], which gives relative uncertainties within 15–20%.
| Wire | FWHM (110) in deg | a (Å) | c (Å) | Tc (K) | ΔTw(K) | x |
|---|---|---|---|---|---|---|
| W0 | 0.45 | 3.0858 | 3.5250 | 37.5 | 0.7 | 0 |
| W10-B | 0.49 | 3.0817 | 3.5265 | 36.0 | 1 or 2 | 0.010 |
| W10 | 0.53 | 3.0798 | 3.5243 | 35.2 | 0.7 | 0.016 |
| W5-n | 0.80 | 3.0684 | 3.5248 | 34.5 | 13.6 | 0.051 |
Considering the full width at half-maximum (FWHM) values of the reflexion peaks, it is observed that the FWHM of the (001) peaks remain constant for all wires, while the (110) peaks become broader for doped wires, with higher width for W5-n (see table 1). The similarities in FWHM of the (002) peak and the differences for the (110) one in the wire with larger C doping is a strong indication of an increase of crystallographic disorder or a reduction of crystallinity, but it also may be due to segregations of phases with different C content or to lack of homogeneity in the doped grains.
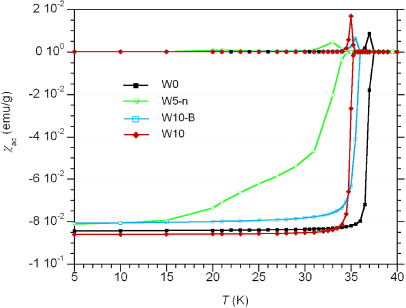
χac(T) measurements have been used to determine the critical temperatures, Tc, and to analyse the sharpness of the superconductor to normal state transitions in all wires. The measured χac(T) curves are shown in figure 2. The Tc-values of the wires, determined by the onset of diamagnetism by χ'(T), have been collected in table 1. They decrease from 37.5 K for the undoped wire (W0) down to 34.5 K for W5-n, and are in good agreement with the expected variations from the estimated C substitution [27].
Figure 2. In-phase and out-of-phase components of the magnetic ac susceptibility χac(T) of the wire superconducting cores.
Download figure:
Standard image High-resolution imageThe transition widths, ΔTw, defined as the temperature interval from 90% to 10% of χ'(5 K) (i.e., of complete diamagnetism), are also collected in table 1. Both W0 and W10 wires have the same value ΔTw = 0.7 K for all annealing conditions, while, for the W10-B wire, ΔTw = 2 K for final annealing at 700 ° C × 2 h, and the value decreases to ΔTw = 1 K for 670 ° C × 5 h. Wire W5-n shows a very wide transition, ΔTw = 13.6 K, which likely indicates the presence of important amounts of non-superconducting phases in this wire.
The FESEM micrographs on transverse cross-sections of these wires with different magnifications are displayed in figure 3. The overall transverse cross-section of W10, displayed in figure 3(a), shows the porous microstructure of the core, typical of in situ PIT wires. The surrounding Fe sheath shows reactivity with B in a layer of thickness 5–8 μm, which is similar for all fabricated wires and cannot be seen in this low-magnification micrograph. There are voids of several micrometres, left by Mg when it diffuses into the B particles, and MgB2 regions quite uniformly distributed. W10 wires present very homogeneous MgB2 phase compositions for all annealing conditions, as may be seen in the enlarged micrograph of figure 3(b), which is similar to the microstructure observed on undoped wire W0.
Figure 3. FESEM (AsB) images obtained on polished cross-sections of wire W10 annealed at 670 ° C, 5 h for two magnifications: (a) and (b); and wire W10-B for different final annealing conditions: (c) 700 ° C × 2 h and (d) 670 ° C × 5 h.
Download figure:
Standard image High-resolution imageA similar FESEM analysis was performed for W10-B. We observed that in this wire there are large areas of size 250–400 μm without voids, unlike W0 and W10 wires, which show a homogeneous distribution of voids. For annealing condition 700 ° C × 2 h, areas with two-phase compositions are abundant, as seen in figure 3(c). EDX analysis showed that darker grey areas correspond to boron-rich zones, while light grey ones are MgB2. In the overall view of the cross-section of this wire, it is seen that these darker grey zones are more abundant in the centre of this dense large area, while the lighter grey ones are closer to voids. When longer annealing times and slightly lower temperatures are used, 670 ° C × 5 h, more areas with homogeneous phase composition are observed (see figure 3(d)) in agreement with [11], although zones with boron- rich phases still persist. These results indicate that there is a lack of precursor distribution homogeneity in wire W10-B, probably due to the agglomeration of boron powders.
EDX analysis reveals the presence of similar oxygen content for W10 and W10-B, ∼5 at.%, which is slightly higher than for the undoped wire, ∼3.5 at.%. This may be due to the additional oxygen introduced by the carboxylic groups of the oleic acid. Then, the main difference between these wires is the homogeneity, which would be originated in the precursor powders. Oleic acid seems to agglomerate boron particles, adding difficulty to the subsequent homogeneous mixing with Mg powders. Nevertheless, this effect does not appear when oleic acid is added to the Mg + B mixture, as in W10.
The FESEM analysis of wire W5-n (not shown) displays phases different to the above-reported ones, and EDX analysis reveals the presence of regions with very high oxygen content (up to 15–20 at.%). Moreover, a considerable amount of carbon should be present as secondary phases, since just ∼5 at.% of carbon coming from oleic acid has substituted B in MgB2. Nevertheless, since samples were coated with carbon for FESEM observations, it was not possible to quantify it with EDX. These carbonaceous phases will act as weak links between MgB2 superconducting regions, which will be the reason for the large transition width observed in the magnetic susceptibility.
3.2. Influence of soaking of the B or Mg + B powders in oleic acid and initial heat treatments
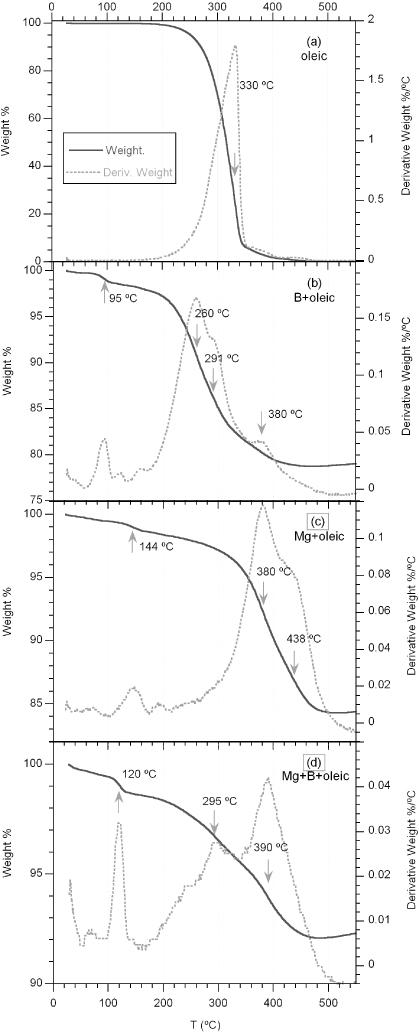
Thermogravimetric analysis has been used to analyse the effect of oleic acid addition on B, Mg, or B + Mg powders. The weight loss (%) and derivative weight—dW(T)—curves as a function of temperature are displayed in figure 4. A needed reference is the weight losses of liquid oleic acid, see figure 4(a), which has a single and smoothed step down, starting at 200 ° C, with maximum of the dW(T) curve at 330 ° C, due to the dragging off of its vapour by the Ar flow, and finishing around the atmospheric pressure boiling point (360 ° C).
Figure 4. Weight loss (%) and corresponding derivative weight (% ° C−1) curves for (a) oleic acid, (b) B + 20 wt% oleic acid, (c) Mg + 20 wt% oleic acid, and (d) Mg + B (1:2 atomic proportions) + 10 wt% oleic acid. (The estimated error is ±1 wt%.) The temperatures at which the derivative weight curves show a maximum are indicated.
Download figure:
Standard image High-resolution imageFor B powders mixed with oleic acid in a proportion similar to the one used in W10-B wire precursors (see figure 4(b)), there is an initial weight loss with maximum of the dW(T) curve at 95 ° C. This is due to the elimination of the water produced by the chemical reaction of oleic acid carboxylic groups (–COOH) with boron oxides present in the B powders to form oleyl borates with water evolution. The dragging of loose bounded oleic acid molecules starts around the same temperatures as liquid oleic acid (∼200 ° C), and dW(T) has a large maximum at 260 ° C. This maximum occurs at lower temperatures than in figure 4(a), due to the small amount of oleic acid. The results of B + oleic are similar to those seen for oleic acid coatings in Co and Ni nanoparticles [26] analysed with TGA and NEXAFS techniques. Those authors also observed thermal desorption of oleic acid at around 240 ° C (i.e., weight loss in TGA and NEXAFS spectra corresponding to oleic acid), whereas at higher temperatures, between 360 and 430 ° C, they observed a weight loss, but also changes in the NEXAFS spectra, with disappearance of C to O double bonds, and only the peak of C=C double bonds in graphitic C remained. Consequently, the peak at 380 ° C in figure 4(b) should be associated with the thermal decomposition (pyrolysis) of oleic acid bounded to B grains producing a carbon-rich coating.
For Mg powders mixed with oleic acid samples (see figure 4(c)), two chemical reactions may take place associated with its carboxylic groups. At low temperatures, the acid may dissolve the MgO precipitates, usually placed at the Mg metallic surfaces, following the chemical reaction 2⋅C17H33–COOH + MgO → [C17H33–COO]2Mg + H2O, which is enhanced at higher temperatures. This effect is marked by a smooth weight loss due to dragging off of created water molecules, peaking at 144 ° C. At higher temperatures, less-bounded oleic acid molecules are dragged off by the Ar flow, but the weight loss rate is much slower than for B powders with oleic. That indicates that oleic acid also reacts with metallic Mg: 2⋅C17H33–COOH + Mg → [C17H33–COO]2Mg + H2. The Mg oleates produced in the above chemical reaction will start to decompose at slightly higher temperatures (pyrolysis), showing a marked maximum at 380 ° C. The pyrolysis is a complex reaction giving oleic acid fragmentation into short-chain hydrocarbons, CO, CO2, and coke, causing fine MgO or MgCO3 precipitates.
For B + Mg powders mixed with oleic acid, at temperatures below the MgB2 formation, the weight losses, displayed in figure 4(d), almost correspond to the addition of the interaction effects observed on B + oleic and Mg + oleic mixtures. There is an enhancement of water losses by the combined effects of the oleic acid on the Mg and B oxides, with a peak at 120 ° C. The decomposition of Mg oleate and oleyl borates produces the peak at 390 ° C. At intermediate temperatures, less-bounded oleic acid molecules are dragged off by the Ar flow.
3.3. Superconducting properties
The inductive critical current densities of the analysed wires have been estimated magnetically from the hysteresis magnetization loop width, ΔM(H) as

where R is the radius of the cylindrical superconducting core and the magnetic field is applied perpendicular to the wire axis. The local distortions of the core cylindrical shape produced during polishing are estimated to be less than 10%. The measurements were done with this orientation in order to have most current paths along the sample's axis, as in transport measurements. Equation (1) is obtained under the assumption of homogeneous induced currents on the length scale of the sample, which will be discussed later. It should be noted that generally it is not valid for fields lower than the penetration field, Hp [28]. However, we have plotted the Jc-values at low fields in order to show the presence of flux jumps.
Figure 5(a) shows a logarithmic representation of Jc as a function of the magnetic field at 5 K for all doped samples for two different annealing conditions: 700 ° C × 2 h and 670 ° C × 5 h. Wire W5-n has the lowest Jc-values, while W10 presents the best results, with slightly higher Jc for lower annealing temperatures and longer times. Wire W10-B has higher critical current densities for treatments at 670 ° C × 5 h than at 700 ° C × 2 h, in agreement with the differences observed on their microstructure.
Figure 5. (a) Critical current densities as a function of the applied field at 5 K deduced from magnetization measurements for all doped samples for two different annealing conditions. (b) Magnetic Jc(H) at 5 and 20 K of wires W0 and W10 annealed at 670 ° C, 5 h. Transport Jc-values at 4.2 K are also shown for comparison.
Download figure:
Standard image High-resolution imageThe comparison between the inductive Jc-values of the optimized doped wire W10 and the undoped one W0 at 5 and 20 K are shown in figure 5(b), together with the transport Jc measured at 4.2 K. There is a large improvement of Jc at medium and high fields at both temperatures for the doped wire W10. The measured transport values are higher than the magnetic ones, especially for the undoped wire W0. A remarkable parallelism between magnetic and transport values is found for W10, although the transport experimental facility limits only just enable one to establish the tendency. It should be noted that the Jc-values of wire W10 are similar to those obtained on Fe tapes fabricated with B powders treated with stearic acid [24].
The analysis of the differences between transport and magnetic Jc-values has been studied by several groups (see, for example, [29–31]). In general, the use of equation (1) is justified by the non-granular behaviour of MgB2 [32]. However, wire inhomogeneities may lead to the appearance of granularity effects, which at high fields are usually indicated by strong downturns of magnetic Jc(H)-curves [29]. When granularity exists, the inductive Jc-values are lower than the transport ones in high fields [29], as in our case. Even so, it must be noted that the high transport critical current values indicate the existence of good connectivity between grains also in these high magnetic fields. In addition to the apparition of granularity, flux creep phenomena would intensify the differences between magnetic and transport values, due to the more restrictive criterion used to define Jc in magnetic measurements compared with the transport ones [31].
The measured transport I–V-curves (V ∝ In) of W0 and W10 wires are steep. For example, at μ0H = 8 T, the estimated n-values are n ∼ 20–24 and n ∼ 30–33 for W0 and W10, respectively. For this field, the transport Jc-values of the doped W10 wires are 4 times higher than for W0. The transport critical currents of wire W10-B are much lower, by approximately one order of magnitude, than their corresponding magnetic values (for example transport Jc = 1.3 × 107 and 6 × 107 A m−2 at 8 T and 5 T, respectively, were measured at 4.2 K). Moreover, its I–V-curve is smooth and shows a resistive tail, in agreement with previous indications of non-homogeneous phase formation on this wire. Similar results are obtained for pre-annealing in vacuum or in Ar.
For the wire with best superconducting properties (W10), a preliminary analysis of the influence on Jc of the annealing conditions at the different steps of wire manufacturing has also been done. First, no relevant difference was observed for wires W10 manufactured with annealing at 400 ° C or at 550 ° C during drawing, although the former seems to get slightly higher Jc-values. Second, in order to obtain the optimum pre-annealing conditions of the precursor, we prepared a wire (with precursors: Mg + 2B + 10 wt% oleic acid), which was drawn up to 2.5 mm diameter without any heat treatment of the precursors or during drawing. Three pieces of 1 cm length were cut and heat treated at 400, 450, or 550 ° C, respectively, for 1 h in Ar atmosphere, and cooled down to room temperature. These pieces were chosen to be short enough to allow the vapours generated during the pre-annealing to get out easily. Afterwards, these three pieces were sealed and annealed at 700 ° C × 2 h in vacuum. The highest magnetic Jc was obtained for the first one. For this reason, the precursors of wire W10 were pre-annealed at 400 ° C × 1 h before wire manufacturing.
4. Conclusions
A new source of C to fabricate C-doped Fe/MgB2 composite wires by oleic acid addition has been used. This produces enhanced critical currents at 20 and 4.2 K in medium and high magnetic fields without deterioration in low fields. The best results correspond to W10 wires, which use mixed Mg + B powders soaked in oleic acid and annealed at 400 ° C to eliminate the excess before wire conformation. These wires have 1.6 at.% of B substitution by C, a Tc decrease to 35.2 K, and improved critical current densities, with good correspondence between transport and magnetic Jc, and good homogeneity.
W10-B wires, which use oleic acid soaked B powders annealed at 500 ° C before mixing with Mg, have smaller B by C substitution (x ≈ 0.010) and slightly lower magnetic Jc(H)-values, but show lower homogeneity, probably due to caking of oleic acid soaked B powders before mixing.
Doping with oleic acid without heat treatment of the precursors before wire processing to eliminate excess increases the C substitution to x = 0.05, but results in poor superconductivity properties, probably because of the large amount of secondary carbonaceous phases and oxides. In this way, a wide transition of the χ'(T) curve and low Jc-values, even at low magnetic fields, have been obtained.
Thermogravimetric analysis of the chemical reactions between oleic acid with precursor boron or magnesium powders has proven the formation of oleyl borates and Mg oleate, respectively, during heat treatments at temperatures below oleic acid's boiling point (360 ° C). The pyrolysis of these salts and of the remnant oleic acid (physically or chemically bonded to the surface of B and Mg grains) produces active carbon sources at temperatures above 380 ° C, which facilitate the B by C substitution when the MgB2 lattice is formed, but also provides the oxygen for final MgO precipitates.
Acknowledgments
Funding of this research by Spanish MINECO and the European FEDER Program (Project MAT2011-22719) and by Gobierno de Aragón (Research group T12) is gratefully acknowledged. The authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación-SAI, Universidad de Zaragoza.