Abstract
In the event of an accidental release of radioactive elements from a nuclear power plant, it has been shown that the radionuclides contributing the most to long-term exposure are 134Cs and 137Cs. In the case of nuclear power plant fallout, with subsequent intake of radionuclides through the food chain, the internal absorbed dose to target tissues from protracted intake of radionuclides needs to be estimated. Internal contamination from food consumption is not caused by a single intake event; hence, the committed equivalent dose, calculated by a dose coefficient or dose per content function, cannot be easily used to calculate the cumulative absorbed dose to relevant target tissues in the body. In this study, we calculated updated absorbed dose rate coefficients for 134Cs and 137Cs based on data from the International Commission on Radiological Protection (ICRP) on specific absorbed fractions. The absorbed dose rate coefficients are provided for male and female adult reference phantoms, respectively, assuming a steady-state distribution of Cs that we calculated from the ICRP biokinetic model for Cs. With these coefficients, the absorbed dose to the listed target tissues, separately and to the total body, are related to the number of nuclear transitions (time-integrated activity) in each listed source region. Our new absorbed dose rate coefficients are given for the complete set of target tissues and have not been presented before. They are also provided for aggregated categories of organs to facilitate epidemiological studies.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In the event of an accidental release of radioactive elements from a nuclear power plant or in case of a radioecological transfer from a nuclear weapon fallout, it has been shown that the long-lived radionuclides 134Cs (T1/2 = 2.1 years) and 137Cs (T1/2 = 30.1 years) contribute the most to the cumulative internal effective dose (e.g. UNSCEAR 2000). To estimate the risk of cancer from exposure to ionising radiation from these radionuclides, the concept of lifetime attributable risk (LAR) has been introduced (NRC 2006). However, to calculate LAR, the absorbed dose to various body tissues/organs needs to be estimated both from external sources as well as from sources within the body. To enable calculation of organ-absorbed doses, Snyder et al (1975) published organ absorbed dose rate coefficients for more than 110 selected radionuclides (S-coefficients), relating the absorbed dose in a target tissue to the total number of nuclear transitions (time-integrated activity) in a given source region.
For radiation protection purposes, the assessment of internal radiation dose is generally based on estimations of intake of a given radionuclide by inhalation or ingestion that can be estimated from, e.g. ambient measurements or bioassays. For the latter, a biokinetic model describing the time-dependent transfer of the radionuclide in the body is required to derive the intake. The internal radiation dose, expressed as the committed effective dose, can then be calculated as the product of the inhaled or ingested activity and a dose coefficient (units Sv Bq−1) for the radionuclide and route of intake. Recently, the International Commission on Radiological Protection (ICRP) published a series of reports that provide the committed effective dose per content functions in terms of committed effective dose per activity content in, e.g. the total body or excreta at a given time after intake (ICRP 2012, 2016b, 2017, 2019). The biokinetic behaviour of the radionuclides is thus embedded in the dose per content functions, which enable a direct estimation of the committed effective dose from whole body counting or bioassays (ICRP 2015, 2016b, 2017, 2019). Furthermore, ICRP has provided extensive data files, permitting the committed equivalent dose to be estimated for target tissues (ICRP 2016a, supplementary material (available online at stacks.iop.org/JRP/41/1213/mmedia)).
The committed equivalent dose due to intake of a radionuclide is defined as the integral of the equivalent dose rate to a given target tissue over a period of 50 years (or to the age of 70 years for children), following a single intake of that radionuclide (ICRP 2015). This computation includes the total exposure to the target tissue from activity distributed over a number of source regions, where the time-dependent biokinetic distribution and excretion is determined by the biochemical properties as well as by the physical half-life of that radionuclide. The equivalent dose is calculated by multiplying the absorbed dose by a weighting factor, which for beta and gamma radiation equals 1 (ICRP 2007). Thus, the numerical values of the equivalent dose and absorbed dose are equal, provided only beta and gamma radiation are considered. If an aim is to compute the time-integrated absorbed dose to a given target tissue from a given source region, this cannot be derived simply by applying the dose coefficients, because (a) the committed equivalent dose is restricted for a fixed integration time, and (b) the equivalent dose to a target tissue includes the contribution from several source regions. In order to determine the absorbed dose to a given target tissue from the activity in the total body, the contribution from each of the source regions to a given target tissue have to be known, taking into account the distribution of the radionuclide among source regions.
In the case of a release from a nuclear power plant or the fallout from a nuclear or radiological weapon, with subsequent intake of radionuclides through the food chain, the internal absorbed dose to target tissues from the continuous intake of radionuclides needs to be estimated. Of note, in calculating LAR, one of the crucial parameters is the time-integrated absorbed dose to a target tissue from a protracted intake of the fission products 134Cs and 137Cs (e.g. US EPA 1999, Andersson et al 2017, Rääf et al 2020). The internal contamination is then not caused by a single intake event, and hence the committed equivalent dose, calculated by a dose coefficient or dose per content function, cannot easily be used to calculate the absorbed dose to relevant target tissues in the body over a continuous time period.
Instead, a coefficient relating the absorbed dose in a given target tissue to the accumulated activity in a given source region is needed. Such coefficients were published by Snyder et al (1975), relating the absorbed dose in a target tissue to the number of nuclear transitions (absorbed dose per accumulated activity) in a source region. The software DCAL (Manabe et al 2010) also provides this data. With a continuous intake of 134Cs and 137Cs, an equilibrium distribution between various body tissues in humans will emerge, and then these coefficients can be interpreted as the absorbed dose rate in a target tissue per activity in a source region, regardless of the biokinetic fate of a given Cs radionuclide. However, the total absorbed dose to a particular target tissue is most often the sum of dose contributions from multiple surrounding source regions. These S-coefficients by Snyder et al (1975) have proved useful when calculating the absorbed dose to the thyroid following the Chernobyl nuclear power plant accident and when calculating LAR by applying an S-coefficient for the total body (Rääf et al 2019, 2020). The absorbed fractions used in the calculations by Snyder et al (1975) were based on computations using the 70 kg unisex Committee on Medical Internal Radiation Dose phantom equipped with both male and female sex organs (Snyder et al 1969), with absorbed dose rate coefficients of 33 and 50 (nGy yr−1) Bq−1 for whole-body absorbed dose from a homogeneous total body content of 137Cs and 134Cs, respectively. Attempts to account for different body sizes when calculating the internal dose from radiocaesium have been made by Falk et al (1991), using a methodology presented by Leggett et al (1984). However, the format of the work by Leggett et al (1984) makes it laborious to translate this method into modern-day phantom tissue specifications. Moreover, for computing LAR to the 11 and 13 cancer-risk-related tissues listed for males and females, respectively, from US EPA (2011), the computations are limited to a subset of organs in the body, because Snyder et al (1975) only report absorbed dose rate coefficients for a few target tissues, ignoring, e.g. brain, breast, and prostate. However, absorbed fractions based on the ICRP computational adult (>18 years) phantoms for males and females separately are now available (ICRP 2009, 2016a), and an update of absorbed dose rate coefficients would therefore be both feasible and warranted. The new phantoms also allow the expansion of the number of target tissues from 19 in Snyder et al (1975) to 43 in ICRP publication 133 (2016a).
Moreover, epidemiological cancer research into the effects of protracted low-dose radiation exposure has long been hampered by the lack of methods for estimating internal dose to organs in the body, often relying only on the external absorbed dose and ignoring the contribution from the internal dose. However, internal absorbed doses delivered to a selection of nine organs from diagnostic nuclear medicine procedures have been estimated, followed by a calculation of excess relative risk of cancer in those organs and estimation of the population attributable fraction of cancer (Marant-Micallef et al 2019). Also, in the Techa River Dosimeter System, separate organs/tissues were selected by calculating organ-specific absorbed doses for several nuclides, but epidemiological studies only used the total body dose from incorporated radionuclides (e.g. Degteva et al 2009, 2019, Krestinina et al 2017).
To enable epidemiological studies of site-specific cancer induction from protracted internal exposures of radiocaesium to various organs, this study aimed to derive updated absorbed dose rate coefficients for 134Cs and 137Cs, improved by sex-specific model parameters, and to relate the absorbed dose in each target tissue and the total body to the number of nuclear transitions (time-integrated activity) in a source region. Related to this aim, target tissues have been translated into anatomical tissues/organs to further facilitate epidemiological studies of cancer sites in occupationally exposed groups or environmental exposure to members of the public.
2. Materials and methods
The absorbed dose in a target tissue, rT, from radioactive decays in a source region, rS, is directly proportional to the number of nuclear transitions in the source region (Bq s), the energy emitted per transition, E, and the fraction of the emitted energy that is absorbed in (imparted to) the target tissue. Furthermore, the absorbed dose is inversely proportional to the mass of the target tissue. ICRP (2016a) has defined the radiation-weighted S-coefficient, Sw(rT ← rS), as

where the index R denotes the type of radiation (e.g. alpha, beta, or gamma) and index i refers to different energies of radiation of type R (e.g. gamma photons of different energies); YR,i is the relative abundance (yield) of radiation i of type R, emitted per nuclear transition. The fraction of the emitted energy absorbed per mass in the target tissue is given by the specific absorbed fraction (SAF), Φ(rT ← rS, ER,i ), and wR is the radiation weighting factor for radiation of type R. The value of Sw(rT ← rS) thus represents the equivalent dose to target tissue T per nuclear transition in source region S (Sv Bq−1 s−1) or the equivalent dose rate per activity (Sv s−1 Bq−1). If only electrons and photons are considered, the radiation weighting factor equals 1 for both types of radiation (ICRP 2007), and the absorbed dose per nuclear transition (Gy Bq−1 s−1) or the absorbed dose rate per activity (Gy s−1 Bq−1) is numerically equal to Sw.
The values of SAFs (kg−1) for photons, alpha particles, electrons, and neutrons, as well as the masses for reference source and target tissues, have been published for the adult reference phantoms by the ICRP as electronic data files (ICRP 2016a, supplementary data). When calculating absorbed dose rate coefficients in this work, the radiation types and energies given in table 1 were considered when extracting SAFs from the electronic data files; for beta radiation, the energies and yields of the beta particles are given by the beta spectra (ICRP 2008). The extracted SAF's are derived by linear interpolation in the electronic data files.
Table 1. Mean energies, ER,i (MeV), for radiation emitted in the decay of 134Cs, 137Cs, and 137mBa, respectively. Data from ICRP (2008, supplementary material; DECDATA software). 'NA' stands for 'not applicable.'.
| Auger | Conversion | Beta | Gamma | ||
|---|---|---|---|---|---|
| Radionuclide | electrons | electrons | radiation | X-rays | rays |
| 134Cs | 5.369 × 10−4 | 0.6158 | Spectrum | 1.829 × 10−3 | 0.6976 |
| 137Cs | 5.379 × 10−4 | 0.2507 | Spectrum | 1.841 × 10−3 | 0.2835 |
| 137mBa | 5.275 × 10−4 | 0.6303 | NA | 1.754 × 10−3 | 0.6617 |
The product ER,i ·YR,i is defined as the equilibrium absorbed dose quantity Δ (ICRP 2008), and the absorbed dose rate coefficient (S-coefficient) can thus be calculated by
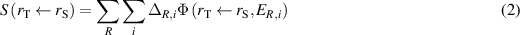
where the radiation types R and energies i consist of those given in table 1 and the values of Δ are given in table 2. For each energy, an intermediate S-value, Si , is also given, calculated as the product of Δ and SAF, i.e.

Table 2. Values of Δ in Gy kg nt−1 (or Gy kg Bq−1 s−1) for radiation emitted in the decay of 134Cs, 137Cs, and 137mBa, respectively. Data from ICRP (2008, supplementary material; DECDATA software). 'nt' denotes 'nuclear transition.' 'NA' stands for 'not applicable'.
| Auger | Conversion | Beta | |||
|---|---|---|---|---|---|
| Radionuclide | electrons | electrons | radiation | X-rays | Gamma rays |
| 134Cs | 1.167 × 10−17 | 1.092 × 10−15 | Spectrum | 4.610 × 10−17 | 2.491 × 10−13 |
| 137Cs | 3.531 × 10−22 | 1.341 × 10−20 | Spectrum | 1.402 × 10−21 | 2.634 × 10−19 |
| 137mBa | 1.063 × 10−16 | 1.036 × 10−14 | NA | 4.087 × 10−16 | 9.513 × 10−14 |
For beta radiation, Si is derived by weighting the energy of the beta particles with the respective yield. For 137Cs, the contribution to the S-coefficients from electrons and photons is calculated by summing the contributions from the decay of 137Cs and the de-excitation of the metastable 137mBa. The S-coefficients are then calculated by summing the contribution from electrons and photons for each radionuclide.
The S-coefficients for the absorbed dose to a target tissue per nuclear transition or, equivalently, the absorbed dose rate per activity in the total body are listed in Snyder et al (1975); these coefficients include the situation where the total body is both a source region and a target tissue. To derive the steady-state distribution of caesium in the body, the ICRP biokinetic model for caesium (ICRP 2017) and the ICRP human alimentary tract model (ICRP 2006) were solved for a continuous intake of 1 Bq d−1.
The compartment model depicted in figure 1 was implemented in the modelling tool Ecolego (AFRY, Sweden). Transfer coefficients for the systemic part of the model (light grey shading) were taken from ICRP Publication 137 (ICRP 2017) and Legget et al (2003) (urinary bladder contents → urine); transfer coefficients for the alimentary tract were taken from ICRP Publication 100 (ICRP 2006) (adult male). Following uptake of 1 Bq d−1 to the plasma compartment, the activity in each compartment in the model was integrated until a steady-state activity was reached and the distribution was then given by dividing the steady-state activity in each compartment by the total activity in all compartments. These calculations were made for both caesium isotopes.
Figure 1. The systemic model for caesium, redrawn from ICRP (2017). Compartments with darker shading are parts of the ICRP human alimentary tract model (ICRP 2006). Reproduced with permission from ICRP (2017), by SAGE Publications, Ltd.
Download figure:
Standard image High-resolution imageHowever, not all source regions for which SAF values are given are included in the biokinetic model for caesium. The model also includes two compartments, denoted Other 1 and Other 2, which correspond to the remaining soft tissues in the body; Other 2 in the model represents a small component of very long-term retention (ICRP 2017). The fraction of caesium distributed in the source regions not depicted in the ICRP biokinetic model was estimated by identifying source regions that could be described as soft tissue. These source regions are listed in table S3 in the supplementary material. The fraction of caesium contained in the compartments Other 1 and Other 2 (table S2 in supplementary material) was distributed over these regions, weighted by their mass in relation to the total mass of the regions identified as 'Other.'
According to these calculations, an equilibrium in 137Cs body burden is achieved after about 500 days. This value is similar also for 134Cs. Thus, for a total body burden of 1 Bq, when equilibrium is achieved for either 134Cs or 137Cs, the fraction fj
of the activity in each of the listed source regions (ICRP 2016a), rS,j
, could be found. The absorbed dose rate  to a target tissue rT,i
caused by nuclear transitions in all source regions is then given by
to a target tissue rT,i
caused by nuclear transitions in all source regions is then given by
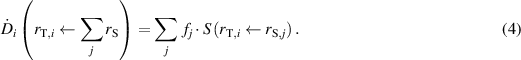
To compare our results with the coefficients given by Snyder et al (1975) for the total body, a corresponding relationship between equilibrium whole-body activity and whole-body dose is calculated. However, the absorbed dose rate to the total body cannot be calculated by summing the absorbed dose rates to the listed target tissues. Instead, the mean energy imparted to each target tissue per time,  , is calculated by multiplying the absorbed dose rate coefficient by the target tissue mass (ICRP 2016a). The absorbed dose rate to the total body is then found by summing the mean energy imparted to each target tissue per time for all target tissues and dividing by the total mass of the target tissues.
, is calculated by multiplying the absorbed dose rate coefficient by the target tissue mass (ICRP 2016a). The absorbed dose rate to the total body is then found by summing the mean energy imparted to each target tissue per time for all target tissues and dividing by the total mass of the target tissues.
It is possible to aggregate target tissues into broader categories of organs to facilitate epidemiological studies by following the same procedure as described above for the absorbed dose to the total body. The mean energy imparted to the tissues to be aggregated is summed and subsequently divided by the total mass of the aggregated tissues.
The obtained S-coefficients can also be used to determine the corresponding absorbed dose rate coefficients for the radiation-sensitive organs specified by BEIR, EPA, and de Gonzalez et al (2012) for translating average organ-absorbed doses from steady-state internal contamination of radiocaesium into organ-specific LAR. To be consistent with the model defined in Rääf et al (2020), assuming a weight dependence suggested by Falk et al (1991) and a uniform radiocaesium distribution according to Snyder et al (1975), we also normalised the revisited absorbed dose rate coefficients in this study to that of the total body (total body to total body) to yield the so-called kOrgan,int-factors. This factor is defined in Rääf et al (2020) as the ratio between organ-absorbed dose and the average whole-body absorbed dose incurred by a uniformly distributed internal contamination of 134Cs and 137Cs, respectively.
3. Results
The calculated absorbed dose rate coefficients are given in tables S1(a)–(d) in the supplementary material for 134Cs adult male, 137Cs adult male, 134Cs adult female, and 137Cs adult female reference phantoms, respectively.
The consequence of using only the primary photon energy in the calculations of Δ was studied by assuming Compton-scattered photons at 0.4 MeV from the isomeric transition in 137mBa and the corresponding value of the SAF. It was found that, in most cases, the calculated S-coefficients were equal (within 5%), and in some cases, the S-coefficients based on the primary photon energy overestimated the absorbed dose by up to a factor of two (e.g. testes ← thyroid).
The absorbed dose to a target tissue per nuclear transition in the total body, including the situation where the total body is both a source region and a target tissue, was calculated based on the steady-state body burdens of 134Cs and 137Cs obtained from the previously mentioned model by ICRP (2006, 2017). Table S2 in the supplementary material gives the computed steady-state caesium content in % of total body, as well as the reference distribution published by ICRP (2017) for comparison. This reference distribution is given for an adult male only, and the biokinetic model does not explicitly include the reproductive organs, which are included in the compartment 'Other.' The resulting distribution among source regions included in 'Other' is given in table S3 in the supplementary material.
The absorbed dose rates,  , to target tissues, rT,i
, were calculated by accounting for nuclear transitions which, according to the biokinetic model, occur in all source regions due to distribution of radionuclide activity in the body after intake of 1 Bq (equation (4)). The absorbed dose rates for the 134Cs adult male, 137Cs adult male, 134Cs adult female, and 137Cs adult female, respectively, are compared with the S-coefficients for the total body as a source organ, as reported by Snyder et al (1975), and are given in table S4 in the supplementary material. Generally, the agreement is within ±30%.
, to target tissues, rT,i
, were calculated by accounting for nuclear transitions which, according to the biokinetic model, occur in all source regions due to distribution of radionuclide activity in the body after intake of 1 Bq (equation (4)). The absorbed dose rates for the 134Cs adult male, 137Cs adult male, 134Cs adult female, and 137Cs adult female, respectively, are compared with the S-coefficients for the total body as a source organ, as reported by Snyder et al (1975), and are given in table S4 in the supplementary material. Generally, the agreement is within ±30%.
The absorbed dose rate coefficients for total body ← total body are given in tables 3 and 4 in the supplementary material. Table S5 in the supplementary material provides the values of mean energy imparted to each target tissue per time and the tissue masses used in the calculations.
Table 3. Mean energy imparted rate,  , to all target organs from all source regions (total body ← total body), total mass of target tissues, and resulting absorbed dose rate coefficient for total body ← total body S(TB ← TB). The values for imparted energy and mass are derived by summing column data in table S5, and S is given by
, to all target organs from all source regions (total body ← total body), total mass of target tissues, and resulting absorbed dose rate coefficient for total body ← total body S(TB ← TB). The values for imparted energy and mass are derived by summing column data in table S5, and S is given by  /mTarget tissues.
/mTarget tissues.
| S(TB ← TB) | |||
|---|---|---|---|
| Reference phantom |
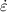 (J s−1) (J s−1) | mTarget tissues (kg) | (Gy s−1 Bq−1) |
| Adult male—134Cs | 9.253 × 10−14 | 59.55 | 1.55 × 10−15 |
| Adult male—137Cs | 6.450 × 10−14 | 59.55 | 1.08 × 10−15 |
| Adult female—134Cs | 9.202 × 10−14 | 49.71 | 1.85 × 10−15 |
| Adult female—137Cs | 6.424 × 10−14 | 49.71 | 1.29 × 10−15 |
Table 4. Internal absorbed dose rate coefficients for males and females according to classification by BEIR VII (NRC 2006) and National Cancer Institute (NCI) and code for classification of disease, ICD-7 (WHO 1957).
| Classification by | Absorbed dose rate coefficient (Gy s−1) | kOrgan,int | ||||
|---|---|---|---|---|---|---|
| BEIR VII + NCI | Cancer sites | ICD-7 | 134Cs | 137Cs | 134Cs | 137Cs |
| (a) Males | ||||||
| Organ-specific radiation-associated cancer | Oral cavity and pharynx a | 1400, 1401, 1408, 1409, 1410, 1417, 1418, 1419, 1420, 1425, 1426, 1428, 1429, 143, 144, 146, 147, 148, 1450, 1457, 1458, 1459 | 9.848 × 10−16 | 5.322 × 10−16 | 0.634 | 0.491 |
| Oesophagus | 1500, 1508, 1509 | 1.203 × 10−15 | 5.583 × 10−16 | 0.774 | 0.516 | |
| Stomach | 151, 1510, 1511, 1518, 1519 | 1.247 × 10−15 | 7.481 × 10−16 | 0.803 | 0.691 | |
| Colon (right + left) b | 1530, 1531, 1532, 1533, 1534, 1536, 1538, 1539 | 1.312 × 10−15 | 7.708 × 10−16 | 0.844 | 0.712 | |
| Liver | 1550 | 1.409 × 10−15 | 8.294 × 10−16 | 0.907 | 0.766 | |
| Gallbladder | 1551 | 1.242 × 10−15 | 5.928 × 10−16 | 0.799 | 0.547 | |
| Rectum c | 1540, 1541, 1548 | 1.599 × 10−15 | 9.051 × 10−16 | 1.029 | 0.836 | |
| Pancreas | 157 | 1.529 × 10−15 | 9.632 × 10−16 | 0.984 | 0.889 | |
| Lung d | 1620, 1621 | 1.552 × 10−15 | 1.020 × 10−15 | 0.999 | 0.942 | |
| Prostate | 177 | 1.588 × 10−15 | 7.140 × 10−16 | 1.022 | 0.659 | |
| Kidneys | 1800, 1809 | 1.552 × 10−15 | 9.139 × 10−16 | 0.999 | 0.844 | |
| Urinary bladder | 1810, 1816 | 1.397 × 10−15 | 6.362 × 10−16 | 0.899 | 0.587 | |
| Central nervous system e | 1921, 1930, 1931, 1938, 1939 | 7.733 × 10−16 | 5.114 × 10−16 | 0.498 | 0.472 | |
| Thyroid | 194 | 1.149 × 10−15 | 5.498 × 10−16 | 0.739 | 0.508 | |
| Leukaemia f | 2040, 2044, 2047, 2049, 2050, 2051, 2059, 2060, 2061, 2069, 2070, 2071, 2072, 2073, 2079 | 1.836 × 10−15 | 1.183 × 10−15 | 1.181 | 1.092 | |
| Other radiation-associated cancer g | Remainder | All other ICD-7 codes | 1.893 × 10−15 | 1.477 × 10−15 | 1.218 | 1.364 |
| Not radiation-associated cancer h | Male breast | 170, 1701, 1702, 1707, 1708, 1709 | 1.697 × 10−15 | 1.062 × 10−15 | 1.092 | 0.981 |
| Lymphoma | 2001, 2002, 2003, 201, 2021, 2022 | |||||
| Other leukaemia | 2024, 203, 2041, 208, 209 | |||||
| Total cancer | All sites | 140–209 | 1.554 × 10−15 | 1.083 × 10−15 | 1.000 | 1.000 |
| (b) Females | ||||||
| Organ-specific radiation-associated cancer | Oral cavity and pharynx a | 1400, 1401, 1408, 1409, 1410, 1417, 1418, 1419, 1420, 1425, 1426, 1428, 1429, 143, 144, 146, 147, 148, 1450, 1457, 1458, 1459 | 1.116 × 10−15 | 6.094 × 10−16 | 0.603 | 0.472 |
| Oesophagus | 1500, 1508, 1509 | 1.576 × 10−15 | 7.460 × 10−16 | 0.852 | 0.577 | |
| Stomach | 151, 1510, 1511, 1518, 1519 | 1.568 × 10−15 | 9.214 × 10−16 | 0.847 | 0.713 | |
| Colon (right + left) b | 1530, 1531, 1532, 1533, 1534, 1536, 1538, 1539 | 1.819 × 10−15 | 1.013 × 10−15 | 0.983 | 0.784 | |
| Liver | 1550 | 1.721 × 10−15 | 1.042 × 10−15 | 0.930 | 0.807 | |
| Gallbladder | 1551 | 1.632 × 10−15 | 7.798 × 10−16 | 0.881 | 0.604 | |
| Rectum c | 1540, 1541, 1548 | 2.001 × 10−15 | 1.089 × 10−15 | 1.081 | 0.843 | |
| Pancreas | 157 | 1.916 × 10−15 | 1.189 × 10−15 | 1.035 | 0.920 | |
| Lung d | 1620, 1621 | 1.889 × 10−15 | 1.254 × 10−15 | 1.021 | 0.970 | |
| Breast | 170, 1701, 1702, 1707, 1708, 1709 | 9.128 × 10−16 | 4.799 × 10−16 | 0.493 | 0.371 | |
| Uterus | 171, 172, 174 | 1.792 × 10−15 | 8.220 × 10−16 | 0.968 | 0.636 | |
| Ovaries | 175, 1750, 1751, 1758, 1759 | 1,935 × 10−15 | 8,799 × 10−16 | 1.045 | 0.681 | |
| Kidneys | 1800, 1809 | 1.999 × 10−15 | 1.150 × 10−15 | 1.080 | 0.890 | |
| Urinary bladder | 1810, 1816 | 1.608 × 10−15 | 7.609 × 10−16 | 0.869 | 0.589 | |
| Central nervous system e | 1921, 1930, 1931, 1938, 1939 | 8.657 × 10−16 | 5.741 × 10−16 | 0.468 | 0.444 | |
| Thyroid | 194 | 1.522 × 10−15 | 7.633 × 10−16 | 0.822 | 0.591 | |
| Leukaemia f | 2040, 2044, 2047, 2049, 2050, 2051, 2059, 2060, 2061, 2069, 2070, 2071, 2072, 2073, 2079 | 2.273 × 10−15 | 1.396 × 10−15 | 1.228 | 1.080 | |
| Other radiation-associated cancer i | Remainder | All other ICD-7 codes | 2.705 × 10−15 | 2.246 × 10−15 | 1.461 | 1.739 |
| Not radiation-associated cancer j | Lymphoma | 2001, 2002, 2003, 201, 2021, 2022 | 2.111 × 10−15 | 1.271 × 10−15 | 1.140 | 0.983 |
| Other leukaemia | 2024, 203, 2041, 208, 209 | |||||
| Total cancer | All sites | 140–209 | 1.851 × 10−15 | 1.292 × 10−15 | 1.000 | 1.000 |
a O-mucosa + ET2-bas + Tongue + S-glands. b RC-stem + LC-stem. c RS-stem. d Bronch-bas + Bronch-sec + Bchiol-sec + AI. e Brain. f R-marrow. g SI-stem + ET1-bas + Endost-BS + Eye-lens + P-gland + Ht-wall + Adrenals + Ureters + Testes + Skin + Muscle. h Breast + LN-ET + LN-TH + Tonsils + Thymus + Spleen + LN-Sys + R-marrow. i SI-stem + ET1-bas + Endost-BS + Eye-lens + P-gland + Ht-wall + Adrenals + Ureters + Skin + Muscle. j LN-ET + LN-TH + Tonsils + Thymus + Spleen + LN-Sys + R-marrow.
Table 4 presents the corresponding coefficients for the organs specified by BEIR VII and the National Cancer Institute (NCI 2020), as well as the kOrgan,int-factors. The former kOrgan,int-factors ranged from 1 to 1.14 for 137Cs and 1.04 to 1.27 for 134Cs. However, using instead a steady-state distribution combined with the recent ICRP anatomical phantom geometry, the values for 137Cs now range from 0.47 to 1.36 and 0.37 to 1.74 for males and females, respectively. For 134Cs, the corresponding ranges are now 0.50–1.22 and 0.47–1.46, respectively.
3.1. Case 1
Assume that measurements by whole-body counting, conducted in an area where individuals continuously ingest radiocaesium-contaminated foodstuff, estimate the whole-body activity in an adult female and an adult male to 500 kBq of 137Cs, respectively. The whole-body burden of the two subjects are hence assumed to be steady-state values. What is the estimated momentary absorbed dose rate to the total body and to the red bone marrow, respectively? Using the absorbed dose rate coefficient given in table 3, the momentary absorbed dose rate to the total body of the female subject can then be estimated as 1.29 × 10−15 × 5.00 × 105 = 0.65 nGy s−1, or 2.3 µGy h−1. For the male subject, the corresponding value is 1.9 µGy h−1. The momentary absorbed dose rate to female red bone marrow can be estimated by the absorbed dose rate to red bone marrow, given in table S5 in the supplementary material, times the whole-body activity, i.e. 1.396 × 10−15 × 5.00 × 105 = 0.70 nGy s−1, or 2.5 µGy h−1. For the male subject, the corresponding dose rate is 2.1 µGy h−1.
3.2. Case 2
LAR of a specific cancer site can be estimated from a radioecological transfer model that relates the initial ground deposition of 137Cs to the average whole-body concentration of 137Cs (such as the one presented by Rääf et al 2006, Isaksson et al 2019). For a ground deposition of 100 kBq m−2, it is predicted that the average 137Cs concentration five years after the fallout is 548 Bq kg−1. For a male subject, aged 30 years with a body weight of 80 kg, this will translate into a body burden of 43.9 kBq. Assume then that this body burden is distributed according to the biokinetic model by ICRP (2017). What is the absorbed dose rate to the colon from 137Cs in this male subject? According to table S5 in the supplementary material, the absorbed dose rate to the colon per Bq 137Cs, averaged over all five defined components of the gastrointestinal tract, will be 7.93 × 10−16 Sv Bq−1. This will lead to an absorbed dose rate of 43.9 × 103 × 7.93 × 10−16 = 34.8 pGy s−1, or 1.10 mGy y−1. Using the risk coefficient of 0.0129 Gy−1 for colon cancer for an adult male of 30 years, an annual absorbed dose of 1.10 mGy delivered to the colon during the 5th year post-fallout will result in an added LAR value of 1.10 × 10−3 × 0.0129 Gy−1 = 1.4 × 10−5, i.e. an added probability of 0.0014% of being diagnosed with colon cancer later in life.
4. Discussion
A direct comparison with the S-coefficients published by Snyder et al (1975) is difficult due to discrepancies regarding the identification of source regions and target tissues for the phantoms used by Snyder et al (1975) and ICRP, respectively, and due to the different approaches to modelling in the two phantoms used. Even if the sources and targets could be reasonably matched in the two data sets, it should be kept in mind that the actual physiological body parts may differ due to the use of different phantoms. However, as previously mentioned, a comparison for the absorbed dose to the total body can be made.
Despite the disagreement regarding absorbed dose rate coefficients for individual target tissues, the values reported by Snyder et al (1975) for total body ← total body, 1.6 × 10−15 Gy s−1 Bq−1 for 134Cs and 1.1 × 10−15 Gy s−1 Bq−1 for 137Cs, are equal to those found in this work for the male phantom. The advantage of our absorbed dose rate coefficients is that we can present them for males and females separately (table 4). However, these coefficients are primarily applicable to adults, and the elaboration of body-size-dependent absorbed dose rate coefficients that can be used for children and adolescents depends on future ICRP work on voxel phantoms for children (ICRP 2015, Schwarz et al 2021a, 2021b). We believe our organ dose coefficients readily can be used to calculate absorbed organ doses after protracted exposure both to occupationally exposed groups and members of the public after an accidental nuclear power plant release.
Table 4 also gives the ICD-7 code for classification of disease (World Health Organization 1957). The advantage of using ICD-7 in classification of cancer is that it easily can be translated into later versions of ICD. The cancer registry at the National Board of Health and Welfare in Sweden began registration of cancer in 1958 and have since the start consistently coded cancer according to ICD-7 along with the version used in that year the cancer case was diagnosed. Therefore, applying ICD-7 it is convenient when creating retrospective cohorts in Sweden and elsewhere. As we intend to use ICD-7 in future cancer epidemiological studies in Sweden after the Chernobyl accident our study on organ dose coefficients directly can be used as a key reference when calculating organ doses followed by dose-response analyses.
5. Summary and conclusions
Based on data on SAFs published by ICRP (2016a), updated values of S-coefficients (absorbed dose rate coefficients) for absorbed dose per nuclear transition (Gy Bq−1 s−1) or, equivalently, absorbed dose rate per activity (Gy s−1 Bq−1) have been calculated for males and females, respectively. The absorbed dose rate coefficients for the total body and tissue/organs have been calculated at a steady-state distribution of 134Cs and 137Cs in the human body, based on the distribution given by the ICRP biokinetic model for Cs. The absorbed dose rate coefficients have been compared with previously published data by Snyder et al (1975), who computed the relationship between the whole-body dose from a uniform distribution of radiocaesium, and adopted to the classification by BEIR VII and de Gonzalez et al (2012). The discrepancies found between our study and Snyder et al (1975) can most probably be attributed to the use of different phantoms when calculating the SAFs. However, the coefficients for the absorbed dose rate to the total body from radiocaesium are equal for male phantoms for both 134Cs and 137Cs. In contrast to earlier reports, the new organ absorbed dose rate coefficients for internal dose include all target tissues listed by ICRP and may therefore be used in comprehensive future epidemiological studies calculating organ-specific cancer risks after protracted exposure of contaminated foodstuffs.
Acknowledgments
None of the authors of this article has any conflict of interest. This work was partially funded by the Uppsala County Council (1040418) through the regional agreement on medical training and clinical research (ALF) with Uppsala University.


