Abstract
Development of magnetic encapsulated microbubble agents that can integrate multiple diagnostic and therapeutic functions is a key focus in both biomedical engineering and nanotechnology and one which will have far-reaching impact on medical diagnosis and therapies. However, properly designing multifunctional agents that can satisfy particular diagnostic/therapeutic requirements has been recognized as rather challenging, because there is a lack of comprehensive understanding of how the integration of magnetic nanoparticles to microbubble encapsulating shells affects their mechanical properties and dynamic performance in ultrasound imaging and drug delivery. Here, a multifunctional imaging contrast and in-situ gene/drug delivery agent was synthesized by coupling super paramagnetic iron oxide nanoparticles (SPIOs) into albumin-shelled microbubbles. Systematical studies were performed to investigate the SPIO-concentration-dependence of microbubble mechanical properties, acoustic scattering response, inertial cavitation activity and ultrasound-facilitated gene transfection effect. These demonstrated that, with the increasing SPIO concentration, the microbubble mean diameter and shell stiffness increased and ultrasound scattering response and inertial cavitation activity could be significantly enhanced. However, an optimized ultrasound-facilitated vascular endothelial growth factor transfection outcome would be achieved by adopting magnetic albumin-shelled microbubbles with an appropriate SPIO concentration of 114.7 µg ml−1. The current results would provide helpful guidance for future development of multifunctional agents and further optimization of their diagnostic/therapeutic performance in clinic.
Export citation and abstract BibTeX RIS
For more information on this article, see medicalphysicsweb.org
1. Introduction
Malignant neoplasms (cancer) has become one of the greatest threats to human beings, thus early diagnosis and effective therapy for cancer have attracted increasing research and clinic interests. With developments in the areas of nano-biotechnology, medical imaging and targeted gene/drug delivery, ultrasound (US)/magnetic resonance imaging (MRI)-guided diagnosis and therapy have been regarded as one of the most promising non-invasive protocols for cancer treatment (Kennedy 2005, Al-Bataineh et al 2012). US imaging is a most popular nonionic imaging modality, which has a long safety record and can provide real-time images at low cost. With the help of US contrast agents (encapsulated microbubbles), the sensitivity and accuracy of cancer detection in clinical applications has been greatly improved (Frauscher et al 2001, Raisinghani and DeMaria 2002, Cosgrove 2006, Postema and Schmitz 2006). Beyond the well-known imaging contrast enhancement effect, an increasing body of literature has also reported that ultrasound contrast agent (UCA) microbubbles (MBs) can serve as good therapeutic agents to facilitate targeted gene/drug delivery, tumor ablation and radiation treatment (Kennedy 2005, Mitragotri 2005, Wu and Nyborg 2008, Czamota et al 2012). With the continuous reformulation of benchmarks and increasing demands in terms of tissue penetration depth, imaging resolution, treatment efficacy and clinic safety, the development and improvement of multifunctional micro/nanometer particle agents for non-invasive cancer diagnosis and treatment have become the focus of interest in broad research areas (Baker 2010, Jin et al 2010, Sailor and Park 2012).
Magnetic nanoparticles can be used as powerful contrast agents for MRI, which is able to provide functional information with high spatial resolution and great soft-tissue contrast (Gupta and Gupta 2005, Indira and Lakshmi 2010, Sandhu et al 2010). Superparamagnetic iron oxide nanoparticles (SPIOs) have been particularly popular in biomedical applications (Soetanto and Watarai 2000, Yang et al 2008, Jin et al 2010, Abbasi et al 2011, Ianos et al 2012), because they possess unique magnetic property, good biocompatibility with tissue and great potential as magnetic nonviral vector. Thus, much effort has been made to design multifunctional diagnostic/therapeutic agents by integrating SPIOs to UCA microbubbles (Yang et al 2009, Chow et al 2010, Park et al 2010, Sailor and Park 2012, Niu et al 2013, Poehomann et al 2014). However, the embedding of SPIOs in MB shell structures would result in the modulation of MB mechanical properties (e.g. size distribution and elasticity), which could significantly affect MB dynamic responses (e.g. acoustic scattering, nonlinear oscillation and cavitation activity) (Borden et al 2005, Wrenn et al 2009, Tung et al 2011). To our knowledge, there is still lack of comprehensive understanding on how the integration of SPIOs in UCA MBs affects their mechanical properties and dynamic performance in US imaging and energy delivery. Therefore, researchers still face great challenges to develop ideal multifunctional agents to satisfy different functional requirements for varied diagnostic/therapeutic applications.
In this work, a type of multifunctional agent was synthesized by loading SPIOs to albumin-shelled perfluorocarbon MBs. In order to obtain an in-depth understanding on the impact of the embedding of magnetic nanoparticles on MB functionalities, the changes of MB mechanical characteristics (e.g. size distribution, shell stiffness), acoustic scattering response, inertial cavitation activity, as well as its capability in ultrasound-enhanced vascular endothelial growth factor (VEGF) transfection, were systemically examined and quantitatively analyzed under different SPIO concentrations.
2. Materials and methods
2.1. Synthesis of perfluorocarbon-filled-albumin-SPIO microbubbles
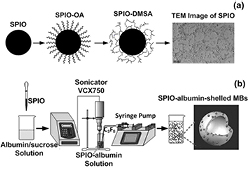
Figure 1 illustrates the procedures in preparing SPIO-albumin-shelled perfluorocarbon MBs (referred to as SPIO-albumin MBs), which includes two major steps: synthesis of SPIOs (figure 1(a)) and assembly of SPIO-albumin MBs (figure 1(b)).
Figure 1. Schematic diagram of multifunctional agent preparation. (a) Monodisperse hydrophobic Fe3O4-OA nanoparticles are firstly synthesized from Fe source. Then, water-soluble SPIOs are obtained via surface double-exchange with DMSA. The transmission electron microscopy (TEM) image illustrates the water-soluble SPIOs remain a monodisperse status with a diameter range between 8–12 nm. (b) Based on a sonicating method, SPIOs are loaded to albumin-shelled perfluorocarbon MBs to fabricate multifunctional imaging and therapeutic agent.
Download figure:
Standard image High-resolution imageFirstly, aqueous solution of FeCl3•6H2O and FeSO4•7H2O was made to serve as a source of iron. Under nitrogen atmosphere to prevent oxidation, concentrated NH3•H2O was added into the iron-containing solution until its pH reached 11.0. Then, oleic acid (OA; Sinopharm Chemical Reagent Co, Ltd, Shanghai, China) was added into the alkaline solution to get mono-disperse hydrophobic Fe3O4-OA nanoparticles. Via a surface double-exchange reaction between OA and meso-2, 3-Dimercaptosuccinic acid (DMSA; Sinopharm Chemical Reagent Co, Ltd, Shanghai, China), mono-disperse water-soluble SPIO (Fe3O4-DMSA) nanoparticles could be obtained and collected through a magnetic separation procedure. After a pH neutralization process, the SPIO solution was dialyzed through the dialysis membranes (Cellu-Sep T3, Membrane Filtration Products Inc, Seguin, Texas, USA) in pure water environment for 3 d to remove excess impurities. The final sample was filtrated through a 0.22 μm membrane and stored at 4 °C. In the second step, SPIO-albumin MBs were synthesized by using a sonicating method similar to that reported by Porter et al (1995). Briefly, 10% bovine serum albumin and 60% sucrose were mixed with a volume ratio of 1:1 in deionized water. A certain amount of SPIOs were then added into the mixed solution. The mixed solution was put in a bell-like glass chamber (9 cm radius and 20 cm height) saturated with perfluorocarbon (C3F8) overnight, then sonicated with an ultrasonic processor (VCX750, Sonics and Materials Inc, Newtown, CT, USA) for 2 min, while a syringe pump (LEGATO 270, KD Scientific Inc Holliston, MA, USA) was used to continuously inflate C3F8 gas. The ultrasonic processor worked at the burst mode with an on/off ratio of 3:1, a working frequency of 20 kHz and an intensity of 300 W. Then, SPIO-albumin MBs were centrifuged and then washed to eliminate free SPIOs. Finally, the upper layer of MB suspensions was collected for subsequent assessments. The detailed description of synthesis procedures can be found in the supplementary data (stacks.iop.org/PMB/59/226729/mmedia).
2.2. 3D Topography observations of microbubbles using atomic force microscopy
High-resolution 3D topography images were constructed using Multimode 8 atomic force microscopy (AFM) with a NanoScope V controller (Bruker Corporation, Billerica, MA, USA) (Vinogradova 2004). SCANASYST-AIR cantilevers with a nominal spring constant of 0.4 N m−1 were used for imaging purpose. The cantilever has a pyramid tip with a side angle of 17.5° and a nominal radius of 2 nm. During the imaging processes, the cantilevers were made to oscillate vertically at 5% below their natural resonant frequency and move in a raster mode within a particular region of interest. The resonant frequency of the SCANASYST-AIR cantilever was about 70 kHz. Prepared microbubble samples were diluted 10 times, dropped onto a fresh mica plate and placed at room temperature for 30 min. All measurements were taken in a water environment on the mica plate. The scanning was performed within a square region (5 × 5 μm) at a scanning rate of 0.5 Hz, with a pixel resolution of 256 × 256. At least ten bubbles were examined for each sample. Through post-processing of raw data with NanoScope Analysis software (Version 1.40, Bruker Co, Billerica, MA, USA), 3D topography images were finally constructed for tested MBs.
2.3. Assessments of microbubble stiffness based on AFM force-displacement measurements
The stiffness of SPIO-albumin MBs was interrogated under the ScanAsyst-contact mode of MultiMode 8 AFM system, with a ramp rate of 0.5 Hz. Microbubble samples were diluted 100 times, dropped on the fresh mica plate and then mechanically interrogated by 40 nm Au/15 nm Ti coated tipless cantilevers (MLCT-O10, Bruker Corporation, Billerica, MA, USA) with nominal spring constants (kc). Prior to measurements, spring constant calibration was performed on the mica plate. The spring constant kc and deflection sensitivity were calibrated to be 0.0228 N m−1 and 58.91 nm V−1, respectively. At least 3 force-displacement curves (no permanent deformation) were acquired for each bubble to ensure experimental reproducibility. The bright-field microscopy images were taken firstly to ensure that the cantilever was parallel to the mica plate and aligned to MB central axis. The effective spring stiffness of individual microbubbles (km) was assessed by measuring the deflection error of AFM cantilever during compression (Sboros et al 2006, Chen et al 2013). During each measurement, the compression force (F) was applied to the tested microbubble through the cantilever and the deflection error-z curve was recorded as raw data. The deflection error, d = F/kc, represented the deflection distance if the cantilever contacted at a hard surface, while the value of z corresponded to actually measured cantilever deflection distance. Thus, the effective deformation of the bubble was calculated as dMB = z–d. The measured d versus. z curves were automatically converted to F versus. dMB curves by using the NanoScope Analysis software and then the effective stiffness of individual microbubbles could be obtained by applying linear regression fit to the force-deformation (F versus. dMB) curve.
2.4. In vitro MRI assessments
To ascertain the ability of SPIO-albumin MBs for MRI contrast enhancement, T1- and T2-weighted MR images were captured. A 3 T MRI scanner (Magnetom Trio, Siemens, München, Germany) was used to conduct all MRI studies. A 12-channel head coil was used for radiofrequency (RF) transmission and reception. For each sample, 1 ml SPIO-albumin MBs taken from individual groups were diluted 15 times. Since the effective SPIO concentrations in these four tested groups were measured to be 0, 19.6, 114.7 and 292.0 μg ml−1, the molar concentrations of iron (Fe) element in the diluted samples were calculated to be 0, 0.01688, 0.0985 and 0.252 mM, respectively. In addition to SPIO-albumin MB solutions, four SPIO solution samples with corresponding Fe molar concentrations were also prepared for comparison. Each sample solution was placed into a 5 ml centrifugal tube for MR scanning. The longitudinal and transverse relaxivities (i.e. r1 and r2) of SPIO-albumin microbubbles and SPIOs were assessed by acquiring series of T1- and T2-weighted images, respectively. The detailed parameters used for MRI assessments can be found in the supplementary data (stacks.iop.org/PMB/59/226729/mmedia).
2.5. In vitro US imaging assessments
A polyacrylamide gel phantom (10 × 10 × 10 cm3) was made to provide mimic tissue background in US image. A custom-made cubic chamber (3 × 3 × 3 cm3) with thin polythene membranes acting as acoustic windows was embedded in the center of the gel phantom. A portable US imaging instrument (Terason T3000, Teratech Corporation, Burlington, MA, USA) with a curved abdomen probe (5C2A, 2–5 MHz working frequency) was used to acquire US images. Each measurement was repeated 5 times. The gray-scale image intensity was calculated in each ROI, using MATLAB software.
2.6. US-facilitated VEGF165 transfection and passive cavitation detection system
All VEGF165 delivery experiments were performed in an acrylic tank filled with degassed water. The US exposure apparatus and passive cavitation detection (PCD) system is similar to that used by Zhang et al (2013). An arbitrary waveform generator supplied 1 MHz sinusoidal pulses at fixed 20-cycle pulse length and 250 Hz pulse repetition frequency (PRF), with varied acoustic peak negative amplitudes (p-) of 0 (sham), 0.05, 0.2, 0.5 or 1.0 MPa. The output signals from the waveform generator were amplified through an RF power amplifier with a fixed gain of 50 dB, which were used to drive a 1 MHz self-made focused source transducer. The focused transducer was comprised of an air-backed, 1.375 inch-diameter piezoelectric (PZT) disk (APC 880, APC International Ltd, Mackeyville, PA, USA) attached to an aluminum-focusing lens with a radius of 9.2 cm curvature and approximately 6.6 cm focal distance (−6 dB and focal width of ~8 mm). A plastic test tube of 10 mm diameter, 0.5 mm thick and 50 mm length filled with sample suspension (the liquid depth of the suspension was ~16 mm) was capped by a custom-built rubber stopper that was used as an acoustic absorber to minimize the effect of standing wave and then sealed with parafilm to minimize bacterial contamination. The test tube was aligned axially with the source transducer so that the center of the suspension was situated at the focal region.
A self-made 5 MHz single-element non-focused PZT transducer (a diameter of 11 mm and a bandwidth of 2–8 MHz), used for PCD measurements, was located perpendicularly to the 1 MHz focused transducer so that its natural focus was orthogonal to that of the source transducer. Co-focusing of the transmitter and receiver transducers and the in situ pressure calibration was performed by using the NTR needle hydrophone (TNU001A, NTR Systems, Inc, Seattle, WA, USA) with a 30 dB preamplifier (HPA30, NTR Systems, Inc, Seattle, WA, USA). The acoustic scattering from bubbles and acoustic emission from inertial cavitation in the focal volume was collected by the 5 MHz transducer and digitized by an oscilloscope. The recorded PCD waveforms were stored in a personal computer for subsequent signal processing using MATLAB. LabView program was used to control the waveform generator and the oscilloscope. The IC 'dose' (ICD) was quantified for each measured PCD signal to assess the cumulated IC energy over a specific US exposure duration. The detailed protocol used to quantify ICD can be found elsewhere (Tu et al 2006).
Human Embryonic Kidney (HEK) 293 T cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen Corp, Carlsbad, CA, USA) supplemented with 10% Fetal bovine serum (FBS; Invitrogen Corp, Carlsbad, CA, USA), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Invitrogen Corp, Carlsbad, CA, USA) and incubated at 37 °C in a humidified atmosphere containing 5% CO2. The cells were harvested using Trypsin-EDTA and resuspended in phosphate-buffered saline before US exposure experiments. In accordance with the method described in previous work (Zhang et al 2013), 8 μg of plasmid pEGFP-N1-VEGF165 was rapidly added into branched polyethylenimine (bPEI; 25 KDa molecular weight; Sigma-Aldrich, St. Louis, MO, USA) solution to produce bPEI:VEGF165 complexes in a pH 7.4 PBS buffer. The mixture was vortexed for 30s and incubated at room temperature for 30 min before adding to the cell suspension. The HEK 293 T cells were harvested using Trypsin-EDTA and resuspended in 0.5 ml PBS at a concentration of 2.0 × 106 cells ml−1. The bPEI:VEGF165 complexes and 1.0 × 107 MBs in 0.5 ml PBS were individually added to the cell suspension. Every sample was briefly vortexed before it was exposed to ultrasound for 20s. The negative control sample was 1.0 × 106 cells added into 1 ml PBS. Five replicates were performed for each treatment. Unless otherwise specified, after incubating the sonicated cells for 2 h at 37 °C and 5% CO2, the cells were centrifuged at 900 rpm (300 g) for 5 min and collected. After washing twice with PBS, the cells were resuspended and cultured in a 6-well plate for another 48 h in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin solution at 37 °C and 5% CO2.
2.7. Quantification of VEGF165 expression by ELISA
The treated HEK 293 cells were collected by centrifugation at 900 rpm for 5 min and washed twice in PBS. Then, the cells were resuspended, cultured in the 6-well plate and incubated for 48 h in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin solution at 37 °C and 5% CO2. After a 48 h incubation period, the supernatant from each sample was harvested and VEGF165 protein levels were measured following the instructions provided in the human VEGF165 Elisa kit (RandD Systems, Minneapolis, MN, USA).
2.8. Assessment of cell viability
Cell viability was measured using the Cell-Counting Kit-8 (CCK-8, Dojindo, Tokyo, Japan). The treated HEK 293 T cells were collected by centrifugation at 900 rpm for 5 min and washed twice in PBS. The cells were resuspended before being put into 96-well plates at a density of 3 × 103 cells well−1. After a 24 h incubation period, CCK-8 solution was added to each well according to the manufacturer's instructions. Plates were incubated for 4 h and the absorbance was measured at 450 nm with a reference wavelength of 600 nm. Cell viability was expressed as the absorbance of the sample divided by that of the negative control.
2.9. Statistical analysis
The data were analyzed and reported as the mean ± standard deviation (SD) using Origin software (OriginLab Co, Northampton, MA, USA). The normality test was performed using Origin software based on Shapiro-wilk test; a t-test was also applied using Origin software to compare results, with p < 0.05 considered to be a statically significant difference.
3. Results
3.1. Physical characterization of SPIO-albumin microbubbles
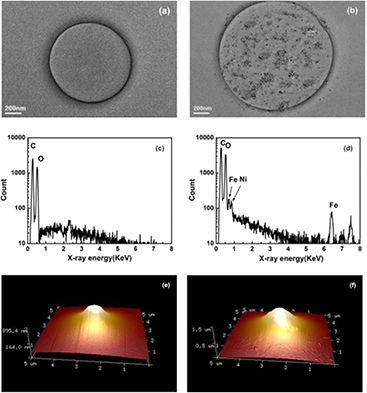
Transmission electron microscopy (TEM) images were taken for MB shells to verify that SPIOs had been successfully coupled to albumin-shelled MBs instead of leaving in the suspension as free residues. The albumin-shelled MB without loading SPIOs shows a uniformly clear surface (see figure 2(a)). However, it is evident in figure 2(b) that lots of nanometer-scale black dots with individual sizes around 8–12 nm exist on the bubble surface, which reveals SPIOs have been successfully integrated to MB shells. Energy dispersive x-ray (EDX) analysis was also applied at 15 kV to further confirm the successful embedding of SPIOs in these MBs. Compared to normal albumin-shelled MBs (figure 2(c)), the EDX spectrum of SPIO-albumin MBs (figure 2(d)) clearly demonstrates the presence of Fe elements in the assembly bubble system. Note that the encapsulated bubble cannot keep the spherical shape, which is explained by the gas inside the bubble being driven out during the fixing and dehydration processes of sample preparation for TEM. Additionally, since SPIO nanoparticles were distributed randomly in the mixed solution during the sonication process, magnetic nanoparticles observed in the TEM image are not uniformly distributed in the bubble shell.
Figure 2. Physical characterization of SPIO-albumin MBs. (a) TEM image of albumin-shelled MBs without the addition of SPIO nanoparticles; (b) TEM image of a SPIO-albumin MB; (c) EDX spectrum analysis of albumin-shelled MBs without SPIO; (d) EDX spectrum analysis of Fe elements in SPIO-albumin microbubbles; (e) typical 3D AFM topography image an albumin-shelled MB without SPIO; and (f) typical 3D AFM topography of a SPIO-albumin MB.
Download figure:
Standard image High-resolution imageTypical 3D topography images of albumin-shelled and SPIO-albumin MBs are illustrated in figures 2(e) and 2(f). The structures shown here are not an ideal spherical shape, but a pyramidal shape, because the interrogating cantilever has a pyramid tip with a nominal radius of 2 nm. The AFM topography is still believed to be capable of revealing the real shape of the upper part of the bubble, since the tip size is much smaller than the bubble. As described in previous work, the roughness of MB top surface can be evaluated with the software (Sboros et al 2006). Meanwhile, the size of a particular MB can be estimated by fitting the MB topography with a sphere, although it might be underestimated because the adhesion with the hard mica plate may affect the sphericity at bubble bottom. In figure 2(e), the diameter of albumin-shelled bubble, it is estimated to be ~1.2 μm with a relatively smooth surface (root-mean-square roughness Rq = 168 nm, roughness average Ra = 144 nm). However, the SPIO-albumin MB (figure 2(f)) exhibits a rougher shell structure (Rq = 328 nm, Ra = 265 nm) with a larger diameter (~1.8 μm), which might result from the embedding of SPIOs onto the bubble shell.
The above magnetization assessment, TEM images and EDX spectra have proven that SPIOs could be successfully loaded to albumin-shelled perfluorocarbon MBs. Furthermore, AFM topography demonstrates that SPIO-albumin MBs have a relatively rougher surface and larger size than that of un-loaded albumin MBs, which indicates the loading of SPIOs might alter the MB shell structure.
3.2. Microbubble size distribution measurements
In the following assessments, SPIO-albumin MB samples were categorized into four groups with varied SPIO concentration (as listed in table 1). In each group, a certain amount of SPIOs (e.g., 0, 0.2, 1.2, or 3.2 mg) were added into 10 ml albumin/sucrose solution (a volume ratio of 1:1) for SPIO-albumin MB preparation and then the effective amount of SPIOs loaded to albumin-shelled MBs was determined by using an Ultraviolet-Visible Spectrometer (UV3600, SHIMADZU CO, Tokyo, Japan). The detailed procedures can be found in the supplementary data (stacks.iop.org/PMB/59/226729/mmedia).
Table 1. The categorization of MB samples with varied SPIO concentrations.
| SPIOs (mg) | Albumin/sucrose (ml) | Effective SPIO concentration in MB suspension (μg ml−1) | |
|---|---|---|---|
| Group 1 | 0 | 10.0 | 0.0 |
| Group 2 | 0.2 | 10.0 | 19.6 |
| Group 3 | 1.2 | 10.0 | 114.7 |
| Group 4 | 3.2 | 10.0 | 292.0 |
A proper size is a key factor in terms of MBs behaving as an effective imaging or therapy agent that can safely pass through pulmonary and vascular circulation system, while proving sensitive US and magnetic responses. Thus, MB size distributions and concentrations were measured under varied SPIO concentrations by using the single particle optical sensing (SPOS) technology; the detailed procedures can be found in the supplementary data (stacks.iop.org/PMB/59/226729/mmedia). For samples in these four groups, MB concentrations were measured to be about 1–2 × 108 bubbles ml−1. An ascending trend can be clearly observed in figure 3 for MB mean diameter with the increasing SPIO concentration. At the highest SPIO concentration (viz, 292.0 μg ml−1) tested here, the mean diameter of SPIO-albumin MBs is about 3.5 μm with 99% smaller than 8 μm, which indicates that these SPIO-albumin MBs are small enough to serve as clinic MB agents.
Figure 3. Microbubble size distribution measured at varied SPIO concentrations.
Download figure:
Standard image High-resolution image3.3. Microbubble stiffness assessments based on AFM
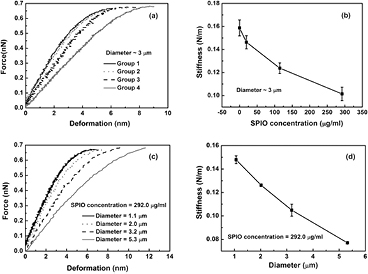
Figure 4(a) shows typical force-deformation curves measured for SPIO-albumin MBs with a diameter of ~3 μm, under varied SPIO concentration. Upon contact with the MB, the cantilever starts to compress the bubble and deflect upwards. Elasticity measurements were performed by adopting a linear elastic assumption for MB deformation based on Hooke's Law. As shown in figure 4(a), with gradually increasing compression force, the force-deformation curves enter a linear region in which the gradient is associated with the effective MB stiffness (Vinogradova 2004, Chen et al 2013). By applying linear regression analyses to the measured force-deformation curves, MB stiffness is plotted as the function of SPIO concentration (figure 4(b)), which indicates that the stiffness of SPIO-albumin MBs decreases with increasing SPIO concentration. The size dependence of MB stiffness was also investigated at a fixed SPIO concentration (i.e. 292.0 μg ml−1 for MBs in Group 4). Figure 4(c) illustrates typical force-deformation curves for MBs with different sizes. It is observed in figure 4(d) that, as the bubble diameter increases from 1 to 5 μm, the MB stiffness decreases from 0.148 ± 0.003 to 0.771 ± 0.001 N m−1.
Figure 4. Mechanical property assessments of SPIO-albumin MBs. (a) and (b) show typical f-s curves and stiffness measured for MBs under varied SPIO concentrations with a diameter of ~ 3 μm. (c) and (d) show typical f-s curves and stiffness measured for MBs at varied diameters with an effective SPIO concentration of ~ 292.0 μg ml−1. Figures 4(b) and (d) plot the results averaged for 10 bubbles in each group.
Download figure:
Standard image High-resolution image3.4. In vitro MRI assessments
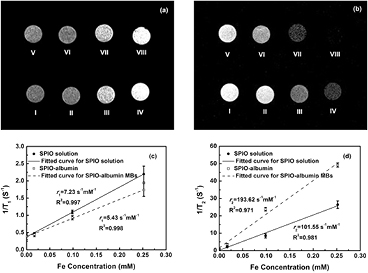
It is well known that SPIOs can be used as effective MRI contrast agents. Thus, the magnetic responses of SPIO-albumin MBs were assessed firstly to evaluate their potentiality as a contrast agent for MRI. T1- and T2-weighted MR images were captured for MB samples with varied SPIO concentration. Typical T1- and T2-weighted images measured for SPIO-albumin MBs and SPIO solutions are illustrated in figures 5(a) and 5(b), respectively. With the increasing Fe concentration, the T2-weighted images obviously turn darker for both SPIO-albumin MBs and SPIOs (figure 5(b)), while only moderate contrast change can be observed in T1-weighted images (figure 5(a)), which suggests that the tested MBs might be more favorable for the contrast enhancement in T2-weighted MRI due to the integration with SPIOs. The longitudinal and transverse relaxation rates (i.e. 1/T1 and 1/T2) are plotted as a function of Fe concentration in figures 5(c) and 5(d). The slope of linear regression fit gives the longitudinal and transverse relaxivities (i.e. r1 and r2) for the tested sample. The ratio r2/r1 determines whether a contrast agent is most suitable for enhancing MR contrast in T1- or T2-weighted image (Tromsdorf et al 2009, Kim et al 2011). According to the results shown in figures 5(d) and 5(c), the r2 and the r2/r1 ratio calculated for the SPIO-albumin MBs are 193.62 S−1 mM−1 and 35.66, respectively, which are significantly enhanced compared to those of SPIOs (about 2 and 2.5 times, respectively). The enhancement of r2 value might be attributed to the synergetic magnetism effect arising from the aggregation and stabilization of multiple SPIOs in MB shells (Berret et al 2006). The higher measured r2/r1 ratio suggests the current SPIO-albumin MBs may be superior negative MRI contrast agents beyond regular SPIOs.
Figure 5. In vitro T1- (a) and T2-weighted (b) MR images for SPIO solutions and SPIO-albumin MB samples with varied Fe concentrations. (I) Degassed water; (II) SPIOs with 0.01688 mM Fe concentration; (III) SPIOs with 0.0985 mM Fe concentration; (IV) SPIOs with 0.252 mM Fe concentration; (V) albumin-shell MBs with 0 mM Fe concentration; (VI) SPIO-albumin MBs with 0.01688 mM Fe concentration; (VII) SPIO-albumin MBs with 0.0985 mM Fe concentration and (VIII) SPIO-albumin MBs with 0.252 mM Fe concentration. Plots of T1-1 (c) and T2-1 (d) versus Fe concentrations for SPIO-albumin MBs and SPIOs. Figures 5(c) and (d) plot the results for five replicated measurements.
Download figure:
Standard image High-resolution image3.5. In vitro US phantom imaging
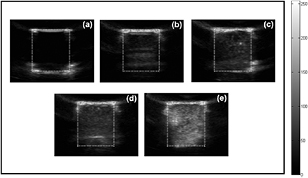
Moreover, US imaging was carried out for the gel phantom in the testing chamber to investigate the impact of the integration of SPIOs on the acoustic scattering capability of SPIO-albumin MBs, which is a dominant factor for their performance in US imaging. To provide significant and distinguishable ultrasound contrast enhancement effect, the MB samples in each group were diluted 30 times and then injected into the testing chamber. Typical US images captured for degassed water and MB solutions with varied SPIO concentrations are shown in figure 6. Compared to degassed water (figure 6(a)), hyperechoic regions can be clearly observed in the testing chamber by adding MB solutions with higher SPIO concentration (figures 6(b)–6(e)). For each sample, the gray-scale image intensity is calculated in the region of interest (ROI) using MATLAB software. The contrast-to-tissue ratio (CTR) is estimated as the ratio of averaged gray-scale intensities between MB and tissue (phantom) regions. According to five repeated measurements, the averaged CTR values are calculated to be −0.59 ± 0.001, 5.89 ± 0.70, 8.97 ± 0.80, 11.04 ± 0.72 and 12.844 ± 0.56 dB for the samples of degassed water and Group 1 to 4, respectively. A negative CTR is discerned for degassed water, which indicates that the tissue-mimicking phantom has stronger echo response than the water. By adding albumin-shelled MBs without SPIOs, significant contrast enhancement effect is achieved. It is noteworthy that, as the effective concentration of SPIOs loaded to MBs increases from 0 to 292.0 μg ml−1, the corresponding CTR is further improved, which suggests that greater US echo intensity can be contributed by MBs integrated with more SPIOs. This will be discussed in detail in the section of discussion.
Figure 6. In vitro US images of (a) degassed water; (b) albumin-shelled MBs; and SPIO-albumin MBs with varied SPIO concentrations of 19.6 (c), 114.7 (d) and 292.0 (e) μg ml−1. The white dotted box indicates the ROI in each image and the gray scale bar represents the dynamic range used in the images.
Download figure:
Standard image High-resolution image3.6. US-facilitated VEGF165 transfection assessments for SPIO-albumin microbubbles
Beyond a dual-modality imaging probe, the proposed SPIO-albumin MBs were also designed as a therapeutic agent. Thus, the enhancement effect of SPIO-albumin MBs on US-facilitated VEGF165 transfection effect was also evaluated in vitro under varied SPIO concentrations. In the experiments, the tested samples were divided into five series: (1) HEK 293 T cells mixed with bPEI:VEGF165 complexes only; and (2)–(5) cells + bPEI:VEGF165 + MBs in Group 1–4 (i.e. SPIO-concentration = 0, 19.6, 114.7 and 292.0 μg ml−1, respectively). 1 MHz US exposures were applied to the tested samples with varying acoustic driving pressures (p-).
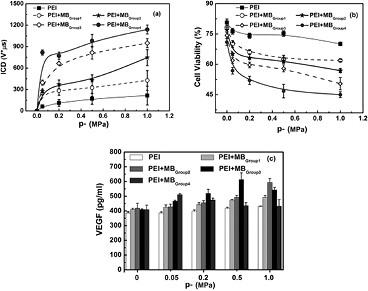
In order to examine the capability of SPIO-albumin MBs on US-facilitated gene transfection, VEGF165 protein secreted into the supernatant of treated cell suspensions was quantitatively measured using ELISA assay and the cell viability was evaluated by CCK-8 assessments. Meanwhile, MB IC energy accumulated during US exposure period was quantified as ICD based on PCD measurements, since MB IC activities have been claimed to play an important role in US-facilitated gene/drug delivery through sonoporation (Miller et al 2002, Van Wamel et al 2006, Zhang et al 2013). The measured ICD, cell viability and VEGF165 transfection efficiency are plotted in figure 7, as a function of p-. It is clearly shown in figure 7(a) that ICD generally increases with the increasing p- and the addition of MBs with higher SPIO concentrations. Cell viability can be expressed as a percentage of the number of viable cells in the experimental samples divided by the number of viable cells in the control samples. As shown in figure 7(b), if sham treatments (i.e. p- = 0) are applied, about a 20% reduction of cell viability might be attributed to the cytotoxicity of bPEI without adding MBs (Fischer et al 1999). However, a slight decline of cell viability (less than 10%) can be observed by adding MBs with increasing SPIO concentration (i.e. from 19.6 to 292.0 μg ml−1). After US exposures, cell viability can be significantly lowered with the increasing p- and SPIO concentration (a maximum reduction of 56.5% is observed under current experiment conditions). Figure 7(c) shows VEGF165 transfection efficiency measured for individual samples. For the sham treatments (p- = 0), VEGF165 transfection is mainly facilitated by bPEI, thus there is no obvious enhancement observed with the addition of MBs. For cells mixed with bPEI:VEGF165 complexes only, US exposures might slightly benefit bPEI:VEGF165 transfection without MB IC activities. As albumin-shelled MBs are added into the suspension (viz, cells mixed with bPEI:VEGF165 complexes with MBs in Group 1), the DNA transfection efficiency can be statistically raised, compared with the samples mixed with bPEI:VEGF165 only. When cells were mixed with bPEI:VEGF165 complexes and MBs in Group 2, the coating of SPIOs on MB shells (effective SPIO concentration = 19.6 μg ml−1) significantly improves US-facilitated VEGF165 transfection. However, as the concentration of SPIOs in MB shells increases to 114.7 μg ml−1 (viz, cells mixed with bPEI:VEGF165 complexes with MBs in Group 3), the transfection efficiency initially increases with the increasing p-, then reaches a peak level of 613.1 ± 46.02 pg ml−1 at p- = 0.5 MPa. As p- is continuously raised to 1.0 MPa, the measured VEGF165 concentration, in turn, drops to 542.9 ± 17.26 pg ml−1. When SPIO concentration further increases to 292.0 μg ml−1 (viz, cells mixed with bPEI:VEGF165 complexes with MBs in Group 4), the measured VEGF165 concentration will increase first, then decrease with the increasing p-, although the peak value appears even earlier at p- = 0.05 MPa. Thus, it is obvious that, compared with cell viability and ICD, much more complicated variation happens in VEGF transfection efficiency assessments.
Figure 7. The dependences of ICD (a), cell viability (b) and VEGF165 transfection efficiency (c) on acoustic driving pressure measured for SPIO-albumin MBs with varied SPIO concentrations. 1 MHz US exposures were performed at fixed 20-cycle pulse length and 250 Hz PRF. The total treatment time for each sample was 20s. The figure plots the results for five replicated measurements.
Download figure:
Standard image High-resolution image4. Discussion
There is a considerable opportunity for multifunctional contrast agents that can offer both accurate early diagnosis and effective clinic treatment simultaneously and it will be even more desirable if the functionalities of these multifunctional agents can be properly modulated by adjusting their structure and mechanical properties with different synthesis protocols. In the present work, a type of multifunctional US/MRI and drug delivery agent was fabricated by coating SPIOs to albumin-shelled perfluorocarbon MBs. The multi-functionality of assembly SPIO-albumin MBs was verified by in vitro US- and MR-imaging as well as US-facilitated gene transfection assessments. Meanwhile, systemic experiments were performed to quantitatively investigate the dependence of MB mechanical characteristics (e.g. size distribution, shell stiffness) and dynamic performances (e.g. acoustic scattering, magnetic response, IC activity and enhancement effects on gene transfection) on the effective SPIO concentration in MB shells.
As shown in figure 3, the size of SPIO-albumin MBs would be enlarged by adding more SPIOs into MB synthesis formula. In fact, the surface tension coefficient of each mixed solution was measured before MB formation by using a BPA-800 P bubble tensiometer (Biolin Scientific AB, Stockholm, Sweden). The measured data showed the surface tension coefficient of the mixed solution increased from 62.0 ± 0.1 to 67.1 ± 0.3 mN m−1 (at 21 °C), as the total amount of SPIOs added into 10 ml albumin shell formula was raised from 0 to 3.2 mg. It is known the formation of bubbles is affected by the chemical composition of the surfactant solution and that bubble size decreases with the decreasing surface tension (Xu et al 2009). Thus, it should be reasonable to expect bigger SPIO-albumin MBs with increasing SPIO concentration.
The encapsulating shell, an additional material present at the gas-liquid interface, plays an important role in stabilizing contrast agent MBs against dissolution and coalescence in bio-physiological environments. The change of physicochemical properties of MB shells has been believed to bring direct impacts on MB mechanical characteristics (e.g. elasticity), which in turn significantly affects MB dynamic behaviors (Borden et al 2005 Sboros et al 2006 Wrenn et al 2009 Tung et al 2011). Before the development of AFM technology, several methods, including backscattering and attenuation measurements (Hoff et al 2000), light scattering (Tu et al 2009) and high-speed optical imaging (Chomas et al 2000), have been used to assess mechanical properties of UCAs. However, due to the limitation of measurement precision for the thin shell materials (e.g. 4 nm lipid shell or 15 nm albumin shell), all of these techniques rely heavily on the numerical fitting of experimental data based on shelled-bubble dynamic models. The application of AFM technology offers a useful tool for direct measurement of MB mechanical parameters (e.g, stiffness) without the need for theoretical models (Sboros et al 2006, Chen et al 2013). By interrogating with AFM cantilevers, the effective stiffness of individual MBs can be quantified by applying linear fits to measured force-deformation curves. The results show that MB stiffness increases with the decreasing SPIO concentration (figure 4(b)) and bubble size (figure 4(d)). The investigation of SPIO-concentration dependence on MB size suggests that smaller SPIO-albumin MBs would be formed at a lower SPIO concentration. For smaller bubbles, the surface area available for domain formation would decrease and the tightly packed structure in its shell with greater curvature might lead to a 'jammed' state of molecules, which could hamper MB compression and then result in a more rigid shell with greater stiffness (Kwan and Borden 2010).
Aiming at dual-modality imaging contrast agents, the acoustic scattering properties of SPIO-albumin MBs was examined based on in vitro US imaging. The results shown in figure 6 demonstrate that, with more SPIOs integrated in MB shells, the contrast-to-tissue ratio could be improved by about 13 dB. For MBs coated with thin shell materials (i.e. shell thickness is much smaller than bubble radius), their dynamic behaviors can be described by Rayleigh-Plesset (RP)-like equations (Hoff et al 2000). The MB scattering cross section (σs), which represents the scattering property of MBs, is calculated as ![${{\sigma}_{s}}=4\pi R{{_{0}^{2}}^{{{\Omega}^{4}}}}\left/\left[{{\left({{\Omega}^{2}}-1\right)}^{2}}+{{\Omega}^{2}}{{\delta}^{2}}\right]\right.$](https://content.cld.iop.org/journals/0031-9155/59/22/6729/revision1/pmb501882ieqn001.gif) (Church 1995, Hoff et al 2000), with
(Church 1995, Hoff et al 2000), with  and
and ![${{\omega}_{0}}=\sqrt{\left[3\gamma {{P}_{0}}+12{}^{{{G}_{s}}{{d}_{s}}}\left/{}_{{R}_{0}}\right.\right]/{{\rho}_{L}}R_{0}^{2}}$](https://content.cld.iop.org/journals/0031-9155/59/22/6729/revision1/pmb501882ieqn003.gif) . Here, R0 is the MB ambient radius; δ is the damping coefficient; γ is the polytropic exponent of the gas; P0 is the hydrostatic pressure in the surrounding liquid; ds is the shell thickness; Gs is the shell shear modulus; ω is the angular frequency of driving signal and ω0 is the linear resonance angular frequency. Figure 3 illustrates that the mean value of MB diameter rises with the increasingly effective SPIO concentration integrated in MBs. According to the above formulas, the increase in R0 would lower the MB resonance frequency ω0 and in turn, enlarge its scattering cross section, which provides theoretical proof for the experimental observations on the enhanced CTR in US images.
. Here, R0 is the MB ambient radius; δ is the damping coefficient; γ is the polytropic exponent of the gas; P0 is the hydrostatic pressure in the surrounding liquid; ds is the shell thickness; Gs is the shell shear modulus; ω is the angular frequency of driving signal and ω0 is the linear resonance angular frequency. Figure 3 illustrates that the mean value of MB diameter rises with the increasingly effective SPIO concentration integrated in MBs. According to the above formulas, the increase in R0 would lower the MB resonance frequency ω0 and in turn, enlarge its scattering cross section, which provides theoretical proof for the experimental observations on the enhanced CTR in US images.
The MRI (figure 5) and US imaging (figure 6) studies have confirmed that, with facile surface modification, the presence of Fe3O4 in MB shell provides enough acoustic and magnetic susceptibility to albumin-shelled UCA MBs to accomplish superb US and MR detectability and sensitivity. To target at multifunctional imaging and drug delivery agents, the therapeutic capability of SPIO-albumin MBs on VEGF165 transfection was also examined in the present work. PEI:VEGF165 complexes were used in this work instead of naked DNA because PEI has been shown to be a relatively efficient non-viral vector and because the synergistic effect of US and PEI on DNA transfection has also been reported in previous work (Deshpande and Prausnitz 2007, Qiu et al 2010, Zhang et al 2013). As reported, US-induced IC activities, which could be dramatically enhanced by the addition of UCA MBs, can transiently enhance cell membrane permeability through sonoporation processes to facilitate the entry of foreign gene/drugs into cells. In order to achieve a better understanding of the mechanisms involved in SPIO-albumin- MB-medicated VEGF165 transfection induced by US exposures, the 'amount' of IC energy cumulated over the US exposure duration (i.e. ICD), cell viability after treatments and effective VEGF165 transfection efficiency were all systemically assessed here at varied p- and SPIO concentration in MB solutions.
For cell samples treated under US sham status, only a slight decrease in cell viability could be observed with the increasing SPIO concentration, which suggests that the current SPIO-albumin MBs are safe for biological application with relatively low cytotoxicity. With US exposures, an increase in p- and SPIO concentration coated in MBs would lead to a significant increase in ICD (figure 7(a)) and decrease in cell viability (figure 7(b)). The enhancement of ICD at higher p- could be simply explained by the greater acoustic energy delivered to the tested samples, which is consistent with previous reports (Qiu et al 2010, Zhang et al 2013). The rising trend of the ICD dependence on SPIO concentration might result from the enlargement of MB size and the reduction of MB stiffness as more SPIOs embedded in the bubble shells. The above MB size (figure 3) and AFM elasticity (figure 4) assessments have shown that, with the lower SPIO concentration, smaller but stiffer MBs might be formed when other conditions remain unchanged. It is feasible that MBs with greater stiffness would have more rigid shells, which could result in an increased resistance to MB collapse and consequently hinder its IC activities. Thus, it is reasonable to expect stronger IC activities for MBs loaded with greater SPIO concentration. Actually, Tung et al (2011) also noticed, in their in vivo blood-brain barrier opening studies, that greater ICD could be obtained with larger bubbles, which is consistent with current observations.
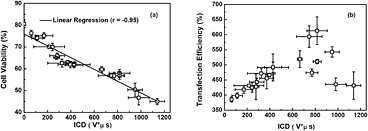
An interesting point worthy of notice is that, unlike nearly monotonic transitions of ICD and cell viability with the increasing p- and SPIO concentration, the variation trend of VEGF165 transfection efficiency seems much more complicated (figure 7(c)). To explore this phenomenon in depth, the correlations between transfection efficiency, cell viability and accumulated IC energy, were studied by analyzing the pooled experimental data. As shown in figure 8(a), significant linear correlation can be observed between the decrease of cell viability and the ICD enhancement. More importantly, it is clearly illustrated in figure 8(b) that, rather than the complex discrepant variations shown in figure 7(c), the DNA transfection efficiency initially increases linearly with the increasing ICD in a certain range. Then, after reaching its peak value at an ICD of about 820 V μs, DNA transfection efficiency tends to drop if the ICD increases continuously. As suggested by previous work, US-facilitated DNA transfection is mainly induced by sonoporation and high correlation could be observed between sonoporation pore size and the measured ICD (Qiu et al 2010). The transient pores generated on the cell membrane should be beneficial for cells to uptake the genetic material, which can lead to the enhancement of DNA transfection efficiency. However, if the IC energy exceeds a certain level (e.g. ICD > 820 V μs), overlarge pores might be generated, which in turn results in unrecoverable damage to cells. As a consequence, the cell viability would be impaired, thereby significantly lowering the DNA transfection efficiency.
Figure 8. Analyses for pooled experimental data. (a) Linear correlation between the cell viability and ICD; (b) the relationship between VEGF165 transfection efficiency and ICD. The figure plots the results for five replicated measurements.
Download figure:
Standard image High-resolution image5. Conclusions
In summary, by coupling SPIOs into albumin-shelled MBs, a type of multifunctional imaging and therapeutic agent was synthesized to take advantage of good biocompatibility, adjustable shell properties, intensified acoustic and magnetic contrast, and significantly enhanced gene transfection efficiency. It might also open new possibilities to develop non-invasive controllable treatment strategies that can simultaneously provide accurate imaging information and efficient therapies in intracerebral hemorrhage diagnosis, gene/drug delivery, cancer treatment, blood-brain barrier opening, etc. Moreover, significant SPIO-concentration-dependence is observed for the mechanical and dynamic responses of these MBs. It shows that, with the increasing SPIO concentration, the MB mean diameter, shell stiffness, US scattering response and acoustic IC activity could be enhanced. However, an optimized US-facilitated VEGF transfection outcome would be achieved by adopting SPIO-albumin MBs with an appropriate concentration of 114.7 µg ml−1. Thus, the structural properties and functionalities of SPIO-albumin microbubbles should be modulated by adjusting the amount of SPIO nanoparticles to satisfy different requirements of particular imaging and therapeutic applications.
It should also be pointed out that the half-life of microbubbles is important for clinical applications of the contrast agents. Limited by our experimental conditions at the current stage, it is not possible for us to quantitatively investigate the half-lime of SPIO-albumin MBs in vivo. In the future, more in vivo studies, including MB half-life and US/MR imaging performance in particular targeted regions, such as liver and kidney, will be performed to achieve even more in-depth understanding of the impact of the integration of SPIOS on MB functionalities and performances in clinics.
Acknowledgements
The authors wish to convey their gratitude for helpful discussions offered by Professor. Gail ter Haar in the Physics Department and the Institute of Cancer Research at Royal Marsden NHS Foundation Trust. This work is partially supported by the National Basic Research Program 973 (grant no. 2011CB707900), National Natural Science Foundation of China (grant no's. 81271589, 81127901, 81227004, 11374155, 11161120324, 11174141, 11274170, 11104140, 1147401 and 11474164), and the National High-Tech Research and Development Program 863 (2012AA022702).